Increased lateral microtubule contact at the cell cortex is sufficient to drive mammalian spindle elongation
- PMID: 28468979
- PMCID: PMC5541847
- DOI: 10.1091/mbc.E17-03-0171
Increased lateral microtubule contact at the cell cortex is sufficient to drive mammalian spindle elongation
Abstract
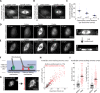
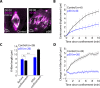
The spindle is a dynamic structure that changes its architecture and size in response to biochemical and physical cues. For example, a simple physical change, cell confinement, can trigger centrosome separation and increase spindle steady-state length at metaphase. How this occurs is not understood, and is the question we pose here. We find that metaphase and anaphase spindles elongate at the same rate when confined, suggesting that similar elongation forces can be generated independent of biochemical and spindle structural differences. Furthermore, this elongation does not require bipolar spindle architecture or dynamic microtubules. Rather, confinement increases numbers of astral microtubules laterally contacting the cortex, shifting contact geometry from "end-on" to "side-on." Astral microtubules engage cortically anchored motors along their length, as demonstrated by outward sliding and buckling after ablation-mediated release from the centrosome. We show that dynein is required for confinement-induced spindle elongation, and both chemical and physical centrosome removal demonstrate that astral microtubules are required for such spindle elongation and its maintenance. Together the data suggest that promoting lateral cortex-microtubule contacts increases dynein-mediated force generation and is sufficient to drive spindle elongation. More broadly, changes in microtubule-to-cortex contact geometry could offer a mechanism for translating changes in cell shape into dramatic intracellular remodeling.
© 2017 Guild et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures



References
-
- Aist JR, Liang H, Berns MW. Astral and spindle forces in PtK2 cells during anaphase B: a laser microbeam study. J Cell Sci. 1993;104:1207–1216. - PubMed
-
- Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, Mitchison TJ, Fang G, Kapoor TM, Salmon ED. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

