Regulation of axonal regeneration following spinal cord injury in the lamprey
- PMID: 28469003
- PMCID: PMC5596130
- DOI: 10.1152/jn.00986.2016
Regulation of axonal regeneration following spinal cord injury in the lamprey
Abstract
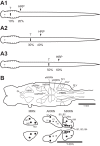
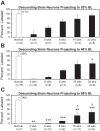
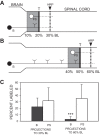
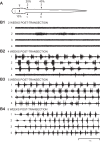
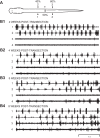
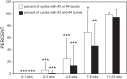
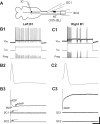
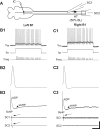
Following rostral spinal cord injury (SCI) in larval lampreys, injured descending brain neurons, particularly reticulospinal (RS) neurons, regenerate their axons, and locomotor behavior recovers in a few weeks. However, axonal regeneration of descending brain neurons is mostly limited to relatively short distances, but the mechanisms for incomplete axonal regeneration are unclear. First, lampreys with rostral SCI exhibited greater axonal regeneration of descending brain neurons, including RS neurons, as well as more rapid recovery of locomotor muscle activity right below the lesion site, compared with animals with caudal SCI. In addition, following rostral SCI, most injured RS neurons displayed the "injury phenotype," whereas following caudal SCI, most injured neurons displayed normal electrical properties. Second, following rostral SCI, at cold temperatures (~4-5°C), axonal transport was suppressed, axonal regeneration and behavioral recovery were blocked, and injured RS neurons displayed normal electrical properties. Cold temperatures appear to prevent injured RS neurons from detecting and/or responding to SCI. It is hypothesized that following rostral SCI, injured descending brain neurons are strongly stimulated to regenerate their axons, presumably because of elimination of spinal synapses and reduced neurotrophic support. However, when these neurons regenerate their axons and make synapses right below the lesion site, restoration of neurotrophic support very likely suppress further axonal regeneration. In contrast, caudal SCI is a weak stimulus for axonal regeneration, presumably because of spared synapses above the lesion site. These results may have implications for mammalian SCI, which can spare synapses above the lesion site for supraspinal descending neurons and propriospinal neurons.NEW & NOTEWORTHY Lampreys with rostral spinal cord injury (SCI) exhibited greater axonal regeneration of descending brain neurons and more rapid recovery of locomotor muscle activity below the lesion site compared with animals with caudal SCI. In addition, following rostral SCI, most injured reticulospinal (RS) neurons displayed the "injury phenotype," whereas following caudal SCI, most injured neurons had normal electrical properties. We hypothesize that following caudal SCI, the spared synapses of injured RS neurons might limit axonal regeneration and behavioral recovery.
Keywords: axotomy; locomotion; neurotrophic factors; reticulospinal neurons.
Copyright © 2017 the American Physiological Society.
Figures
Similar articles
-
Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs.Cells. 2021 Jul 29;10(8):1921. doi: 10.3390/cells10081921. Cells. 2021. PMID: 34440690 Free PMC article.
-
Descending propriospinal neurons mediate restoration of locomotor function following spinal cord injury.J Neurophysiol. 2017 Jan 1;117(1):215-229. doi: 10.1152/jn.00544.2016. Epub 2016 Oct 19. J Neurophysiol. 2017. PMID: 27760818 Free PMC article.
-
Time course of locomotor recovery and functional regeneration in spinal cord-transected lamprey: in vitro preparations.J Neurophysiol. 1994 Aug;72(2):847-60. doi: 10.1152/jn.1994.72.2.847. J Neurophysiol. 1994. PMID: 7983540
-
Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
-
The Lesioned Spinal Cord Is a "New" Spinal Cord: Evidence from Functional Changes after Spinal Injury in Lamprey.Front Neural Circuits. 2017 Nov 6;11:84. doi: 10.3389/fncir.2017.00084. eCollection 2017. Front Neural Circuits. 2017. PMID: 29163065 Free PMC article. Review.
Cited by
-
Spinal cord injury significantly alters the properties of reticulospinal neurons: delayed repolarization mediated by potassium channels.J Neurophysiol. 2023 Nov 1;130(5):1265-1281. doi: 10.1152/jn.00251.2023. Epub 2023 Oct 11. J Neurophysiol. 2023. PMID: 37820016 Free PMC article.
-
Spinal Cord Injury Significantly Alters the Properties of Reticulospinal Neurons: I. Biophysical Properties, Firing Patterns, Excitability, and Synaptic Inputs.Cells. 2021 Jul 29;10(8):1921. doi: 10.3390/cells10081921. Cells. 2021. PMID: 34440690 Free PMC article.
-
Animal Models of Spinal Cord Injury.Biomedicines. 2025 Jun 10;13(6):1427. doi: 10.3390/biomedicines13061427. Biomedicines. 2025. PMID: 40564146 Free PMC article. Review.
-
Neurons Are Not All the Same: Diversity in Neuronal Populations and Their Intrinsic Responses to Spinal Cord Injury.ASN Neuro. 2025;17(1):2440299. doi: 10.1080/17590914.2024.2440299. Epub 2025 Jan 16. ASN Neuro. 2025. PMID: 39819292 Free PMC article. Review.
References
-
- Benes JA. The Effects of Axotomy on the Biophysical Properties of Reticulospinal Neurons in Larval Lamprey (Dissertation). Columbia, MO: University of Missouri, 2006.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

