Developmental roles of tyrosine metabolism enzymes in the blood-sucking insect Rhodnius prolixus
- PMID: 28469016
- PMCID: PMC5443934
- DOI: 10.1098/rspb.2016.2607
Developmental roles of tyrosine metabolism enzymes in the blood-sucking insect Rhodnius prolixus
Abstract
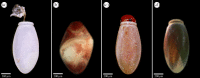
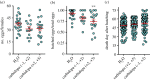
The phenylalanine/tyrosine degradation pathway is frequently described as a catabolic pathway that funnels aromatic amino acids into citric acid cycle intermediates. Previously, we demonstrated that the accumulation of tyrosine generated during the hydrolysis of blood meal proteins in Rhodnius prolixus is potentially toxic, a harmful outcome that is prevented by the action of the first two enzymes in the tyrosine degradation pathway. In this work, we further evaluated the relevance of all other enzymes involved in phenylalanine/tyrosine metabolism in the physiology of this insect. The knockdown of most of these enzymes produced a wide spectrum of distinct phenotypes associated with reproduction, development and nymph survival, demonstrating a highly pleiotropic role of tyrosine metabolism. The phenotypes obtained for two of these enzymes, homogentisate dioxygenase and fumarylacetoacetase, have never before been described in any arthropod. To our knowledge, this report is the first comprehensive gene-silencing analysis of an amino acid metabolism pathway in insects. Amino acid metabolism is exceptionally important in haematophagous arthropods due to their particular feeding behaviour.
Keywords: Chagas disease; Rhodnius prolixus; phenylalanine/tyrosine metabolism; reproduction and development.
© 2017 The Author(s).
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Infanger L-C, Rocheleau TA, Bartholomay LC, Johnson JK, Fuchs J, Higgs S, Chen C-C, Christensen BM. 2004. The role of phenylalanine hydroxylase in melanotic encapsulation of filarial worms in two species of mosquitoes. Insect. Biochem. Mol. Biol 34, 1329–1338. (10.1016/j.ibmb.2004.09.004) - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

