Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis
- PMID: 28469072
- PMCID: PMC5414562
- DOI: 10.1172/jci.insight.86608
Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis
Abstract
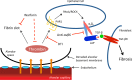
Fibrotic lung disease, most notably idiopathic pulmonary fibrosis (IPF), is thought to result from aberrant wound-healing responses to repetitive lung injury. Increased vascular permeability is a cardinal response to tissue injury, but whether it is mechanistically linked to lung fibrosis is unknown. We previously described a model in which exaggeration of vascular leak after lung injury shifts the outcome of wound-healing responses from normal repair to pathological fibrosis. Here we report that the fibrosis produced in this model is highly dependent on thrombin activity and its downstream signaling pathways. Direct thrombin inhibition with dabigatran significantly inhibited protease-activated receptor-1 (PAR1) activation, integrin αvβ6 induction, TGF-β activation, and the development of pulmonary fibrosis in this vascular leak-dependent model. We used a potentially novel imaging method - ultashort echo time (UTE) lung magnetic resonance imaging (MRI) with the gadolinium-based, fibrin-specific probe EP-2104R - to directly visualize fibrin accumulation in injured mouse lungs, and to correlate the antifibrotic effects of dabigatran with attenuation of fibrin deposition. We found that inhibition of the profibrotic effects of thrombin can be uncoupled from inhibition of hemostasis, as therapeutic anticoagulation with warfarin failed to downregulate the PAR1/αvβ6/TGF-β axis or significantly protect against fibrosis. These findings have direct and important clinical implications, given recent findings that warfarin treatment is not beneficial in IPF, and the clinical availability of direct thrombin inhibitors that our data suggest could benefit these patients.
Keywords: Inflammation; Pulmonology.
Conflict of interest statement
Figures
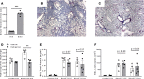
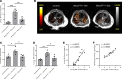
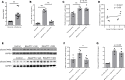

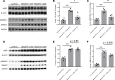

References
-
- Selman M, King TE, Pardo A, American Thoracic Society, European Respiratory Society, American College of Chest Physicians Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

