Serum MicroRNAs Reflect Injury Severity in a Large Animal Model of Thoracic Spinal Cord Injury
- PMID: 28469141
- PMCID: PMC5431108
- DOI: 10.1038/s41598-017-01299-x
Serum MicroRNAs Reflect Injury Severity in a Large Animal Model of Thoracic Spinal Cord Injury
Abstract
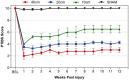
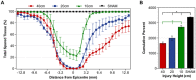
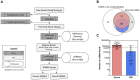
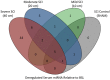
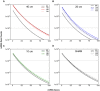
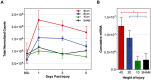
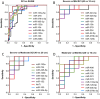
Therapeutic development for spinal cord injury is hindered by the difficulty in conducting clinical trials, which to date have relied solely on functional outcome measures for patient enrollment, stratification, and evaluation. Biological biomarkers that accurately classify injury severity and predict neurologic outcome would represent a paradigm shift in the way spinal cord injury clinical trials could be conducted. MicroRNAs have emerged as attractive biomarker candidates due to their stability in biological fluids, their phylogenetic similarities, and their tissue specificity. Here we characterized a porcine model of spinal cord injury using a combined behavioural, histological, and molecular approach. We performed next-generation sequencing on microRNAs in serum samples collected before injury and then at 1, 3, and 5 days post injury. We identified 58, 21, 9, and 7 altered miRNA after severe, moderate, and mild spinal cord injury, and SHAM surgery, respectively. These data were combined with behavioural and histological analysis. Overall miRNA expression at 1 and 3 days post injury strongly correlates with outcome measures at 12 weeks post injury. The data presented here indicate that serum miRNAs are promising candidates as biomarkers for the evaluation of injury severity for spinal cord injury or other forms of traumatic, acute, neurologic injury.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Bracken MB, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third national acute spinal cord injury randomized controlled trial. National acute spinal cord injury study. JAMA. 1997;277(20):1597–604. doi: 10.1001/jama.1997.03540440031029. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

