Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients
- PMID: 28469156
- PMCID: PMC5431124
- DOI: 10.1038/s41598-017-01387-y
Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine-N-oxide in chronic kidney disease patients
Abstract
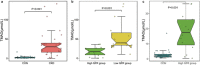
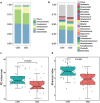
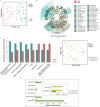
Chronic kidney disease (CKD) patients have an increased risk of cardiovascular diseases (CVDs). The present study aimed to investigate the gut microbiota and blood trimethylamine-N-oxide concentration (TMAO) in Chinese CKD patients and explore the underlying explanations through the animal experiment. The median plasma TMAO level was 30.33 μmol/L in the CKD patients, which was significantly higher than the 2.08 μmol/L concentration measured in the healthy controls. Next-generation sequence revealed obvious dysbiosis of the gut microbiome in CKD patients, with reduced bacterial diversity and biased community constitutions. CKD patients had higher percentages of opportunistic pathogens from gamma-Proteobacteria and reduced percentages of beneficial microbes, such as Roseburia, Coprococcus, and Ruminococcaceae. The PICRUSt analysis demonstrated that eight genes involved in choline, betaine, L-carnitine and trimethylamine (TMA) metabolism were changed in the CKD patients. Moreover, we transferred faecal samples from CKD patients and healthy controls into antibiotic-treated C57BL/6 mice and found that the mice that received gut microbes from the CKD patients had significantly higher plasma TMAO levels and different composition of gut microbiota than did the comparative mouse group. Our present study demonstrated that CKD patients had increased plasma TMAO levels due to contributions from both impaired renal functions and dysbiosis of the gut microbiota.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

