Revisiting antithrombotic therapeutics; sculptin, a novel specific, competitive, reversible, scissile and tight binding inhibitor of thrombin
- PMID: 28469161
- PMCID: PMC5431157
- DOI: 10.1038/s41598-017-01486-w
Revisiting antithrombotic therapeutics; sculptin, a novel specific, competitive, reversible, scissile and tight binding inhibitor of thrombin
Abstract
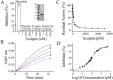
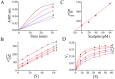
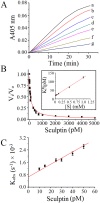
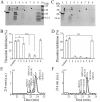
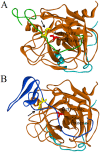
Thrombin is a multifunctional enzyme with a key role in the coagulation cascade. Its functional modulation can culminate into normal blood coagulation or thrombosis. Thus, the identification of novel potent inhibitors of thrombin are of immense importance. Sculptin is the first specific thrombin inhibitor identified in the transcriptomics analysis of tick's salivary glands. It consists of 168 residues having four similar repeats and evolutionary diverged from hirudin. Sculptin is a competitive, specific and reversible inhibitor of thrombin with a Ki of 18.3 ± 1.9 pM (k on 4.04 ± 0.03 × 107 M-1 s-1 and k off 0.65 ± 0.04 × 10-3 s-1). It is slowly consumed by thrombin eventually losing its activity. Contrary, sculptin is hydrolyzed by factor Xa and each polypeptide fragment is able to inhibit thrombin independently. A single domain of sculptin alone retains ~45% of inhibitory activity, which could bind thrombin in a bivalent fashion. The formation of a small turn/helical-like structure by active site binding residues of sculptin might have made it a more potent thrombin inhibitor. In addition, sculptin prolongs global coagulation parameters. In conclusion, sculptin and its independent domain(s) have strong potential to become novel antithrombotic therapeutics.
Conflict of interest statement
The author(s) see no competing financial interests. However, this molecule is submitted for patenting with INPI number BR 102017005783-6.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

