Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers
- PMID: 28469183
- PMCID: PMC5431218
- DOI: 10.1038/s41598-017-01581-y
Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers
Abstract
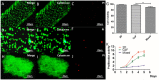
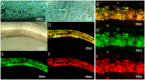
Three-dimensional (3D) bioprinting of living structures with cell-laden biomaterials has been achieved in vitro, however, some cell-cell interactions are limited by the existing hydrogel. To better mimic tumor microenvironment, self-assembled multicellular heterogeneous brain tumor fibers have been fabricated by a custom-made coaxial extrusion 3D bioprinting system, with high viability, proliferative activity and efficient tumor-stromal interactions. Therein, in order to further verify the sufficient interactions between tumor cells and stroma MSCs, CRE-LOXP switch gene system which contained GSCs transfected with "LOXP-STOP-LOXP-RFP" genes and MSCs transfected with "CRE recombinase" gene was used. Results showed that tumor-stroma cells interacted with each other and fused, the transcription of RFP was higher than that of 2D culture model and control group with cells mixed directly into alginate, respectively. RFP expression was observed only in the cell fibers but not in the control group under confocal microscope. In conclusion, coaxial 3D bioprinted multicellular self-assembled heterogeneous tumor tissue-like fibers provided preferable 3D models for studying tumor microenvironment in vitro, especially for tumor-stromal interactions.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






Similar articles
-
Fabrication and validation of an affordable DIY coaxial 3D extrusion bioprinter.Sci Rep. 2025 Jul 2;15(1):22978. doi: 10.1038/s41598-025-06478-9. Sci Rep. 2025. PMID: 40594412 Free PMC article.
-
Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments.Biofabrication. 2018 Jan 12;10(2):024102. doi: 10.1088/1758-5090/aa9d44. Biofabrication. 2018. PMID: 29176035 Free PMC article.
-
Alginate dependent changes of physical properties in 3D bioprinted cell-laden porous scaffolds affect cell viability and cell morphology.Biomed Mater. 2019 Sep 25;14(6):065009. doi: 10.1088/1748-605X/ab3c74. Biomed Mater. 2019. PMID: 31426033
-
3D bioprinting complex models of cancer.Biomater Sci. 2023 May 16;11(10):3414-3430. doi: 10.1039/d2bm02060b. Biomater Sci. 2023. PMID: 37000528 Review.
-
Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine.Int J Mol Sci. 2022 Mar 22;23(7):3432. doi: 10.3390/ijms23073432. Int J Mol Sci. 2022. PMID: 35408789 Free PMC article. Review.
Cited by
-
Advanced tumor organoid bioprinting strategy for oncology research.Mater Today Bio. 2024 Aug 8;28:101198. doi: 10.1016/j.mtbio.2024.101198. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39205873 Free PMC article. Review.
-
Three-dimensional-engineered bioprinted in vitro human neural stem cell self-assembling culture model constructs of Alzheimer's disease.Bioact Mater. 2021 Sep 23;11:192-205. doi: 10.1016/j.bioactmat.2021.09.023. eCollection 2022 May. Bioact Mater. 2021. PMID: 34938923 Free PMC article.
-
The promising rise of bioprinting in revolutionalizing medical science: Advances and possibilities.Regen Ther. 2021 Jun 15;18:133-145. doi: 10.1016/j.reth.2021.05.006. eCollection 2021 Dec. Regen Ther. 2021. PMID: 34189195 Free PMC article. Review.
-
A 3D-Printed Aqueous Drainage Tube with an Expandable Inner Diameter to Accommodate the Intraocular Pressure (IOP) Fluctuations After Glaucoma Surgery.Polymers (Basel). 2025 Jan 5;17(1):118. doi: 10.3390/polym17010118. Polymers (Basel). 2025. PMID: 39795521 Free PMC article.
-
Coaxial Bioprinting of Enzymatically Crosslinkable Hyaluronic Acid-Tyramine Bioinks for Tissue Regeneration.Polymers (Basel). 2024 Aug 30;16(17):2470. doi: 10.3390/polym16172470. Polymers (Basel). 2024. PMID: 39274103 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

