Photoplethysmography for the Assessment of Haemorheology
- PMID: 28469198
- PMCID: PMC5431178
- DOI: 10.1038/s41598-017-01636-0
Photoplethysmography for the Assessment of Haemorheology
Abstract
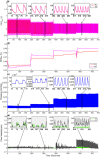
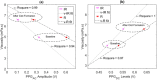
Haemorheology has been long identified as an early biomarker of a wide range of diseases, especially cardiovascular diseases. This study investigates for the first time the suitability of Photoplethysmography (PPG) as a non-invasive diagnostic method for haemorheological changes. The sensitivity of both PPG components (AC and DC) to changes in haemorheology were rigorously investigated in an in vitro experimental setup that mimics the human circulation. A custom-made reflectance PPG sensor, a pressure transducer and an ultrasonic Doppler flowmeter were used to map changes in flow dynamics and optical responses in an arterial model. The study investigated the effect of shear rates by varying fluid pumping frequencies using 4 set-points and the effect of clot formation using a chemical trigger. Both PPGAC amplitudes and PPGDC levels showed significant (p < 0.001) changes during the increase in shear rates and an immediate change after thromboplastin activation. The findings highlight that PPG has the potential to be used as a simple non-invasive method for the detection of blood characteristics, including disaggregation, radial migration and cross-linking fibrin formations. Such capability will enable the assessment of the effects of clotting-activators and anticoagulants (including non-pharmacological methods) and might aid in the early non-invasive assessment of cardiovascular pathologies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







References
-
- Le Dévéhat C, Vimeux M, Khodabandehlou T. Blood rheology in patients with diabetes mellitus. Clin. Hemorheol. Microcirc. 2004;30:297–300. - PubMed
-
- von Tempelhoff G-F, Nieman F, Heilmann L, Hommel G. Association between blood rheology, thrombosis and cancer survival in patients with gynecologic malignancy. Clin. Hemorheol. Microcirc. 2000;22:107–130. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

