Capacity of humic substances to complex with iron at different salinities in the Yangtze River estuary and East China Sea
- PMID: 28469240
- PMCID: PMC5431113
- DOI: 10.1038/s41598-017-01533-6
Capacity of humic substances to complex with iron at different salinities in the Yangtze River estuary and East China Sea
Abstract
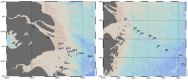
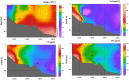
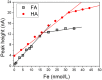
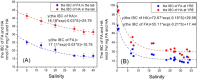
The iron binding capacities (IBC) of fulvic acid (FA) and humic acid (HA) were determined in the salinity range from 5 to 40. The results indicated that IBC decreased while salinity increased. In addition, dissolved iron (dFe), FA and HA were also determined along the Yangtze River estuary's increasing salinity gradient from 0.14 to 33. The loss rates of dFe, FA and HA in the Yangtze River estuary were up to 96%, 74%, and 67%, respectively. The decreases in dFe, FA and HA, as well as the change in IBC of humic substances (HS) along the salinity gradient in the Yangtze River estuary were all well described by a first-order exponential attenuation model: y(dFe/FA/HA, S) = a0 × exp(kS) + y0. These results indicate that flocculation of FA and HA along the salinity gradient resulted in removal of dFe. Furthermore, the exponential attenuation model described in this paper can be applied in the major estuaries of the world where most of the removal of dFe and HS occurs where freshwater and seawater mix.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Influence of humic substances on iron distribution in the East China Sea.Chemosphere. 2018 Aug;204:450-462. doi: 10.1016/j.chemosphere.2018.04.018. Epub 2018 Apr 9. Chemosphere. 2018. PMID: 29679866
-
Terrestrial humic substances in Daliao River and its estuary: optical signatures and photoreactivity to UVA light.Environ Sci Pollut Res Int. 2016 Apr;23(7):6459-71. doi: 10.1007/s11356-015-5876-6. Epub 2015 Dec 2. Environ Sci Pollut Res Int. 2016. PMID: 26627698
-
Salinity-dominated change in community structure and ecological function of Archaea from the lower Pearl River to coastal South China Sea.Appl Microbiol Biotechnol. 2014 Sep;98(18):7971-82. doi: 10.1007/s00253-014-5838-9. Epub 2014 Jun 1. Appl Microbiol Biotechnol. 2014. PMID: 24880629
-
Richness and diversity of helminth communities in the Japanese grenadier anchovy, Coilia nasus, during its anadromous migration in the Yangtze River, China.J Parasitol. 2012 Jun;98(3):449-52. doi: 10.1645/GE-2983.1. Epub 2012 Jan 18. J Parasitol. 2012. PMID: 22257179
-
Trace metals in estuaries in the Russian Far East and China: case studies from the Amur River and the Changjiang.Sci Total Environ. 2014 Nov 15;499:196-211. doi: 10.1016/j.scitotenv.2014.08.015. Epub 2014 Sep 2. Sci Total Environ. 2014. PMID: 25190045
Cited by
-
Trace metal stoichiometry of dissolved organic matter in the Amazon plume.Sci Adv. 2022 Aug 5;8(31):eabm2249. doi: 10.1126/sciadv.abm2249. Epub 2022 Aug 5. Sci Adv. 2022. PMID: 35930637 Free PMC article.
References
-
- Egami F. Origin and early evolution of transition element enzymes. Journ. biochem. 1975;77(6):1165–1169. - PubMed
-
- Raven JA. The iron and molybdenum use efficiencies of plant growth with different energy, carbon and nitrogen sources. New Phytologist. 1988;109(3):279–287. doi: 10.1111/j.1469-8137.1988.tb04196.x. - DOI
-
- Wu J, Sunda W, Boyle EA, Karl DM. Phosphate depletion in the western North Atlantic. Ocean. Science. 2000;289:759–762. - PubMed
-
- Liu X, Millero FJ. The solubility of iron in seawater. Mar. Chem. 2002;77:43–54. doi: 10.1016/S0304-4203(01)00074-3. - DOI
-
- van den Berg CMG. Evidence for organic complexation of iron in seawater. Mar. Chem. 1995;50:139–157. doi: 10.1016/0304-4203(95)00032-M. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

