Neuronal control of peripheral insulin sensitivity and glucose metabolism
- PMID: 28469281
- PMCID: PMC5418592
- DOI: 10.1038/ncomms15259
Neuronal control of peripheral insulin sensitivity and glucose metabolism
Abstract
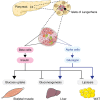
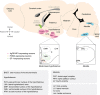
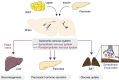
The central nervous system (CNS) has an important role in the regulation of peripheral insulin sensitivity and glucose homeostasis. Research in this dynamically developing field has progressed rapidly due to techniques allowing targeted transgenesis and neurocircuitry mapping, which have defined the primary responsive neurons, associated molecular mechanisms and downstream neurocircuitries and processes involved. Here we review the brain regions, neurons and molecular mechanisms by which the CNS controls peripheral glucose metabolism, particularly via regulation of liver, brown adipose tissue and pancreatic function, and highlight the potential implications of these regulatory pathways in type 2 diabetes and obesity.
Figures
Similar articles
-
Metabolism: A is for adipokine.Nature. 2005 Jul 21;436(7049):337-8. doi: 10.1038/436337a. Nature. 2005. PMID: 16034406 No abstract available.
-
Pancreatic adipocytes mediate hypersecretion of insulin in diabetes-susceptible mice.Metabolism. 2019 Aug;97:9-17. doi: 10.1016/j.metabol.2019.05.005. Epub 2019 May 18. Metabolism. 2019. PMID: 31108105
-
Role of lipids in insulin resistance and type 2 diabetes mellitus development.Nutrition. 1999 Jan;15(1):64-6. doi: 10.1016/s0899-9007(98)00114-2. Nutrition. 1999. PMID: 9918070 No abstract available.
-
Targeting fatty acid metabolism to improve glucose metabolism.Obes Rev. 2015 Sep;16(9):715-57. doi: 10.1111/obr.12298. Epub 2015 Jul 16. Obes Rev. 2015. PMID: 26179344 Review.
-
Exercise and physical training: effects on insulin sensitivity and glucose metabolism.Diabetes Metab Rev. 1986;2(1-2):1-17. doi: 10.1002/dmr.5610020101. Diabetes Metab Rev. 1986. PMID: 3522142 Review. No abstract available.
Cited by
-
The insulin resistant brain: impact on whole-body metabolism and body fat distribution.Diabetologia. 2024 Jul;67(7):1181-1191. doi: 10.1007/s00125-024-06104-9. Epub 2024 Feb 16. Diabetologia. 2024. PMID: 38363340 Free PMC article. Review.
-
Evidence for Compromised Insulin Signaling and Neuronal Vulnerability in Experimental Model of Sporadic Alzheimer's Disease.Mol Neurobiol. 2018 Dec;55(12):8916-8935. doi: 10.1007/s12035-018-0985-0. Epub 2018 Apr 3. Mol Neurobiol. 2018. PMID: 29611103
-
Brain-Derived Neurotrophic Factor as a Potential Mediator of the Beneficial Effects of Myo-Inositol Supplementation during Suckling in the Offspring of Gestational-Calorie-Restricted Rats.Nutrients. 2024 Mar 27;16(7):980. doi: 10.3390/nu16070980. Nutrients. 2024. PMID: 38613013 Free PMC article.
-
The ARCCRABP1 neurons play a crucial role in the regulation of energy homeostasis.Nat Commun. 2025 Mar 8;16(1):2319. doi: 10.1038/s41467-025-57411-7. Nat Commun. 2025. PMID: 40057489 Free PMC article.
-
The acromegaly lipodystrophy.Front Endocrinol (Lausanne). 2022 Sep 13;13:933039. doi: 10.3389/fendo.2022.933039. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36176462 Free PMC article.
References
-
- Wild S., Roglic G., Green A., Sicree R. & King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27, 1047–1053 (2004). - PubMed
-
- Stevens J. et al.. The effect of age on the association between body-mass index and mortality. N. Engl. J. Med. 338, 1–7 (1998). - PubMed
-
- Ezzati M., Lopez A. D., Rodgers A., Vander Hoorn S. & Murray C. J. Comparative Risk Assessment Collaborating G. Selected major risk factors and global and regional burden of disease. Lancet 360, 1347–1360 (2002). - PubMed
-
- Kasuga M., Karlsson F. A. & Kahn C. R. Insulin stimulates the phosphorylation of the 95,000-dalton subunit of its own receptor. Science 215, 185–187 (1982). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

