Treatment Challenges and Survival Analysis of Human Epidermal Growth Factor Receptor 2-positive Breast Cancer in Real World
- PMID: 28469333
- PMCID: PMC5398102
- DOI: 10.4103/0971-5851.203511
Treatment Challenges and Survival Analysis of Human Epidermal Growth Factor Receptor 2-positive Breast Cancer in Real World
Abstract
Context: Advent of trastuzumab has brought tremendous changes in the survival of human epidermal growth factor receptor 2 (Her2)-positive breast cancer patients. Despite the availability of the drug, it is still out of reach for many patients. There is very limited real world data regarding treatment challenges and survival analysis of these patients.
Aims and objectives: Primary objective is disease-free survival (DFS) and secondary objective is overall survival (OS) and toxicity profile.
Statistics: Statistical analysis is done using GraphPad Prism 7.02.
Materials and methods: This is a retrospective study of all patients diagnosed with Her2-positive (Her2+) nonmetastatic invasive breast cancer from January 2007 to December 2013.
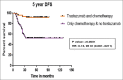
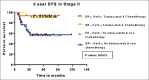
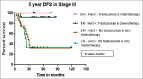
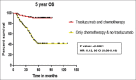
Results: In the period of this study, 885 patients are diagnosed with carcinoma breast, of which 212 are Her2/neu positive (23.9%). Of the 212 patients, only 76 (35.8%) patients received trastuzumab along with chemotherapy. Patients receiving trastuzumab with chemotherapy have longer 5-year DFS compared to those receiving chemotherapy alone, 92% and 52.6%, respectively (P = 0.0001). Five-year OS is 90.5% and 41.7% in those patients who received chemotherapy with and without trastuzumab, respectively (P = 0.0001). Seven patients (9.45%) developed Grade II reversible diastolic dysfunction. Grade II/III peripheral neuropathy due to paclitaxel is the main adverse effect seen in 21 patients.
Conclusion: In spite of improvement in DFS and OS with trastuzumab, the number of patient receiving targeted therapy is very low due to financial constraints which need to be addressed to bridge the gap in survival of Her2+ patients.
Keywords: Breast cancer; India; human epidermal growth factor receptor 2-positive; real world.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Phase II Study of Weekly Paclitaxel with Trastuzumab and Pertuzumab in Patients with Human Epidermal Growth Receptor 2 Overexpressing Metastatic Breast Cancer: 5-Year Follow-up.Oncologist. 2019 Aug;24(8):e646-e652. doi: 10.1634/theoncologist.2018-0512. Epub 2019 Jan 2. Oncologist. 2019. PMID: 30602614 Free PMC article. Clinical Trial.
-
Systemic targeted therapy for her2-positive early female breast cancer: a systematic review of the evidence for the 2014 Cancer Care Ontario systemic therapy guideline.Curr Oncol. 2015 Mar;22(Suppl 1):S114-22. doi: 10.3747/co.22.2322. Curr Oncol. 2015. PMID: 25848335 Free PMC article. Review.
-
Efficacy and tolerability of trastuzumab emtansine in advanced human epidermal growth factor receptor 2-positive breast cancer.Hong Kong Med J. 2018 Feb;24(1):56-62. doi: 10.12809/hkmj176808. Epub 2018 Jan 12. Hong Kong Med J. 2018. PMID: 29326401
-
A phase I trial of ganetespib in combination with paclitaxel and trastuzumab in patients with human epidermal growth factor receptor-2 (HER2)-positive metastatic breast cancer.Breast Cancer Res. 2017 Aug 2;19(1):89. doi: 10.1186/s13058-017-0879-5. Breast Cancer Res. 2017. PMID: 28764748 Free PMC article. Clinical Trial.
-
One year versus a shorter duration of adjuvant trastuzumab for HER2-positive early breast cancer: a systematic review and meta-analysis.Breast Cancer Res Treat. 2019 Jan;173(2):247-254. doi: 10.1007/s10549-018-5001-x. Epub 2018 Oct 13. Breast Cancer Res Treat. 2019. PMID: 30317424
Cited by
-
Neoadjuvant chemotherapy with biosimilar trastuzumab in human epidermal growth factor receptor 2 overexpressed non-metastatic breast cancer: patterns of use and clinical outcomes in India.Ecancermedicalscience. 2021 Mar 19;15:1207. doi: 10.3332/ecancer.2021.1207. eCollection 2021. Ecancermedicalscience. 2021. PMID: 33912232 Free PMC article.
-
Practical consensus recommendations regarding the management of HER2 neu positive early breast cancer.South Asian J Cancer. 2018 Apr-Jun;7(2):102-105. doi: 10.4103/sajc.sajc_111_18. South Asian J Cancer. 2018. PMID: 29721473 Free PMC article.
-
Clinicopathological differences and survival benefit in ER+/PR+/HER2+ vs ER+/PR-/HER2+ breast cancer subtypes.Breast Cancer. 2024 Mar;31(2):295-304. doi: 10.1007/s12282-023-01538-2. Epub 2024 Jan 17. Breast Cancer. 2024. PMID: 38231460
-
Pharmacogenomics and health disparities, are we helping?Front Genet. 2023 Jan 23;14:1099541. doi: 10.3389/fgene.2023.1099541. eCollection 2023. Front Genet. 2023. PMID: 36755573 Free PMC article. Review.
-
Unique challenges and outcomes of young women with breast cancers from a tertiary care cancer centre in India.Breast. 2021 Dec;60:177-184. doi: 10.1016/j.breast.2021.09.008. Epub 2021 Oct 6. Breast. 2021. PMID: 34655887 Free PMC article.
References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. - PubMed
-
- Vaidyanathan K, Kumar P, Reddy CO, Deshmane V, Somasundaram K, Mukherjee G. ErbB-2 expression and its association with other biological parameters of breast cancer among Indian women. Indian J Cancer. 2010;47:8–15. - PubMed
-
- Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–6. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

