Therapeutic Interventions of Cancers Using Intrinsically Disordered Proteins as Drug Targets: c-Myc as Model System
- PMID: 28469390
- PMCID: PMC5392011
- DOI: 10.1177/1176935117699408
Therapeutic Interventions of Cancers Using Intrinsically Disordered Proteins as Drug Targets: c-Myc as Model System
Abstract
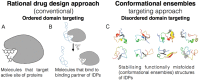
The concept of protein intrinsic disorder has taken the driving seat to understand regulatory proteins in general. Reports suggest that in mammals nearly 75% of signalling proteins contain long disordered regions with greater than 30 amino acid residues. Therefore, intrinsically disordered proteins (IDPs) have been implicated in several human diseases and should be considered as potential novel drug targets. Moreover, intrinsic disorder provides a huge multifunctional capability to hub proteins such as c-Myc and p53. c-Myc is the hot spot for understanding and developing therapeutics against cancers and cancer stem cells. Our past understanding is mainly based on in vitro and in vivo experiments conducted using c-Myc as whole protein. Using the reductionist approach, c-Myc oncoprotein has been divided into structured and disordered domains. A wealth of data is available dealing with the structured perspectives of c-Myc, but understanding c-Myc in terms of disordered domains has just begun. Disorderness provides enormous flexibility to proteins in general for binding to numerous partners. Here, we have reviewed the current progress on understanding c-Myc using the emerging concept of IDPs.
Keywords: Intrinsically disordered proteins; and molecular recognition elements; conformational ensembles; therapeutics and drug development; transcription factors.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Structure-based Inhibitor Design for the Intrinsically Disordered Protein c-Myc.Sci Rep. 2016 Mar 2;6:22298. doi: 10.1038/srep22298. Sci Rep. 2016. PMID: 26931396 Free PMC article.
-
The dark proteome of cancer: Intrinsic disorderedness and functionality of HIF-1α along with its interacting proteins.Prog Mol Biol Transl Sci. 2019;166:371-403. doi: 10.1016/bs.pmbts.2019.05.006. Epub 2019 Jun 8. Prog Mol Biol Transl Sci. 2019. PMID: 31521236 Review.
-
Proteus: a random forest classifier to predict disorder-to-order transitioning binding regions in intrinsically disordered proteins.J Comput Aided Mol Des. 2017 May;31(5):453-466. doi: 10.1007/s10822-017-0020-y. Epub 2017 Apr 1. J Comput Aided Mol Des. 2017. PMID: 28365882 Free PMC article.
-
Proteomic and bioinformatic analysis of a nuclear intrinsically disordered proteome.J Proteomics. 2016 Jan 1;130:76-84. doi: 10.1016/j.jprot.2015.09.004. Epub 2015 Sep 12. J Proteomics. 2016. PMID: 26376097
-
Mechanisms of small-molecule binding to intrinsically disordered proteins.Biochem Soc Trans. 2012 Oct;40(5):1004-8. doi: 10.1042/BST20120086. Biochem Soc Trans. 2012. PMID: 22988855 Review.
Cited by
-
Gain-of-Function Mutations: An Emerging Advantage for Cancer Biology.Trends Biochem Sci. 2019 Aug;44(8):659-674. doi: 10.1016/j.tibs.2019.03.009. Epub 2019 Apr 29. Trends Biochem Sci. 2019. PMID: 31047772 Free PMC article. Review.
-
Discovery of a Pyrazolopyridinone-Based MYC Inhibitor That Selectively Engages Intracellular c-MYC and Disrupts MYC-MAX Heterodimerization.J Med Chem. 2025 Mar 27;68(6):6233-6251. doi: 10.1021/acs.jmedchem.4c02556. Epub 2025 Mar 12. J Med Chem. 2025. PMID: 40077826 Free PMC article.
-
Mechanistic Insights into Zika Virus NS3 Helicase Inhibition by Epigallocatechin-3-Gallate.ACS Omega. 2020 May 4;5(19):11217-11226. doi: 10.1021/acsomega.0c01353. eCollection 2020 May 19. ACS Omega. 2020. PMID: 32455246 Free PMC article.
-
Dual-Inhibitors of N-Myc and AURKA as Potential Therapy for Neuroendocrine Prostate Cancer.Int J Mol Sci. 2020 Nov 5;21(21):8277. doi: 10.3390/ijms21218277. Int J Mol Sci. 2020. PMID: 33167327 Free PMC article.
-
Intrinsically Disordered Proteins: An Overview.Int J Mol Sci. 2022 Nov 14;23(22):14050. doi: 10.3390/ijms232214050. Int J Mol Sci. 2022. PMID: 36430530 Free PMC article. Review.
References
-
- Uversky VN. Functional roles of transiently and intrinsically disordered regions within proteins. FEBS J. 2015;282:1182–1189. - PubMed
-
- Oldfield CJ, Dunker AK. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem. 2014;83:553–584. - PubMed
-
- Jirgensons B. Classification of proteins according to conformation: Die Makromol. Chemie 1966;91:74–86.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

