Analytical Method Validation of High-Performance Liquid Chromatography and Stability-Indicating Study of Medroxyprogesterone Acetate Intravaginal Sponges
- PMID: 28469407
- PMCID: PMC5345923
- DOI: 10.1177/1177390117690152
Analytical Method Validation of High-Performance Liquid Chromatography and Stability-Indicating Study of Medroxyprogesterone Acetate Intravaginal Sponges
Abstract
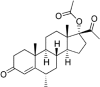
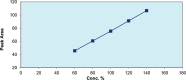
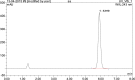
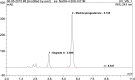
Medroxyprogesterone acetate is widely used in veterinary medicine as intravaginal dosage for the synchronization of breeding cycle in ewes and goats. The main goal of this study was to develop reverse-phase high-performance liquid chromatography method for the quantification of medroxyprogesterone acetate in veterinary vaginal sponges. A single high-performance liquid chromatography/UV isocratic run was used for the analytical assay of the active ingredient medroxyprogesterone. The chromatographic system consisted of a reverse-phase C18 column as the stationary phase and a mixture of 60% acetonitrile and 40% potassium dihydrogen phosphate buffer as the mobile phase; the pH was adjusted to 5.6. The method was validated according to the International Council for Harmonisation (ICH) guidelines. Forced degradation studies were also performed to evaluate the stability-indicating properties and specificity of the method. Medroxyprogesterone was eluted at 5.9 minutes. The linearity of the method was confirmed in the range of 0.0576 to 0.1134 mg/mL (R2 > 0.999). The limit of quantification was shown to be 3.9 µg/mL. Precision and accuracy ranges were found to be %RSD <0.2 and 98% to 102%, respectively. Medroxyprogesterone capacity factor value of 2.1, tailing factor value of 1.03, and resolution value of 3.9 were obtained in accordance with ICH guidelines. Based on the obtained results, a rapid, precise, accurate, sensitive, and cost-effective analysis procedure was proposed for quantitative determination of medroxyprogesterone in vaginal sponges. This analytical method is the only available method to analyse medroxyprogesterone in veterinary intravaginal dosage form.
Keywords: HPLC; intravaginal sponges; medroxyprogesterone; validation.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
A Simple, Fast, Low Cost, HPLC/UV Validated Method for Determination of Flutamide: Application to Protein Binding Studies.Adv Pharm Bull. 2016 Jun;6(2):251-6. doi: 10.15171/apb.2016.034. Epub 2016 Jun 30. Adv Pharm Bull. 2016. PMID: 27478788 Free PMC article.
-
Rapid Development and Validation of Improved Reversed-Phase High-performance Liquid Chromatography Method for the Quantification of Mangiferin, a Polyphenol Xanthone Glycoside in Mangifera indica.Pharmacognosy Res. 2017 Apr-Jun;9(2):215-219. doi: 10.4103/0974-8490.204652. Pharmacognosy Res. 2017. PMID: 28539748 Free PMC article.
-
A Study of Method Development, Validation, and Forced Degradation for Simultaneous Quantification of Paracetamol and Ibuprofen in Pharmaceutical Dosage Form by RP-HPLC Method.Anal Chem Insights. 2014 Nov 18;9:75-81. doi: 10.4137/ACI.S18651. eCollection 2014. Anal Chem Insights. 2014. PMID: 25452691 Free PMC article.
-
Development of a stability-indicating UPLC method for determination of isotretinoin in bulk drug.Pharm Dev Technol. 2019 Feb;24(2):189-198. doi: 10.1080/10837450.2018.1454469. Epub 2018 Apr 12. Pharm Dev Technol. 2019. PMID: 29558234
-
Fast RP-HPLC Method for the Determination of Bisoprolol.Rev Med Chir Soc Med Nat Iasi. 2016 Jul-Sep;120(3):720-6. Rev Med Chir Soc Med Nat Iasi. 2016. PMID: 30152661
Cited by
-
Analytical Quality by Design Approach of Reverse-Phase High-Performance Liquid Chromatography of Atorvastatin: Method Development, Optimization, Validation, and the Stability-Indicated Method.Int J Anal Chem. 2021 Feb 13;2021:8833900. doi: 10.1155/2021/8833900. eCollection 2021. Int J Anal Chem. 2021. PMID: 33628253 Free PMC article.
References
-
- Ganellin CR, Triggle DJ. Dictionary of Pharmacological Agents. Abingdon, UK: Taylor & Francis; 1996.
-
- Index Nominum 2000: International Drug Directory. London, England: Medpharm Scientific Publishers; 2000.
-
- Panay N, Fenton A. Bioidentical hormones: what is all the hype about? Climacteric. 2010;13:1–3. - PubMed
-
- Abecia JA, Forcada F, González-Bulnes A. Pharmaceutical control of reproduction in sheep and goats. Vet Clin North Am Food Anim Pract. 2011;27:67–79. - PubMed
-
- Pietroski ACCA, Brandão FZ, De Souza JMG, Da Fonseca JF. Short, medium or long-term hormonal treatments for induction of synchronized estrus and ovulation in Saanen goats during the nonbreeding season. Rev Bras Zootecn. 2013;42:168–173.
LinkOut - more resources
Full Text Sources
Other Literature Sources