Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects
- PMID: 28469468
- PMCID: PMC5398323
- DOI: 10.1177/1178646917691938
Kynurenine Pathway of Tryptophan Metabolism: Regulatory and Functional Aspects
Abstract
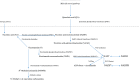
Regulatory and functional aspects of the kynurenine (K) pathway (KP) of tryptophan (Trp) degradation are reviewed. The KP accounts for ~95% of dietary Trp degradation, of which 90% is attributed to the hepatic KP. During immune activation, the minor extrahepatic KP plays a more active role. The KP is rate-limited by its first enzyme, Trp 2,3-dioxygenase (TDO), in liver and indoleamine 2,3-dioxygenase (IDO) elsewhere. TDO is regulated by glucocorticoid induction, substrate activation and stabilization by Trp, cofactor activation by heme, and end-product inhibition by reduced nicotinamide adenine dinucleotide (phosphate). IDO is regulated by IFN-γ and other cytokines and by nitric oxide. The KP disposes of excess Trp, controls hepatic heme synthesis and Trp availability for cerebral serotonin synthesis, and produces immunoregulatory and neuroactive metabolites, the B3 "vitamin" nicotinic acid, and oxidized nicotinamide adenine dinucleotide. Various KP enzymes are undermined in disease and are targeted for therapy of conditions ranging from immunological, neurological, and neurodegenerative conditions to cancer.
Keywords: 3-dioxygenase; indoleamine 2; kynureninase; kynurenine aminotransferase; nicotinamide; tryptophan 2; tryptophan disposition.
Conflict of interest statement
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Disclosures and Ethics The author has provided to the publisher signed confirmation of compliance with legal and ethical obligations including, but not limited to, the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The author has read and confirmed his agreement with the ICMJE authorship and conflict of interest criteria. The author has also confirmed that this article is unique and not under consideration or published in any other publication, and that he has permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
Figures
References
-
- Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6:1–97. - PubMed
-
- Badawy AA-B. Tryptophan metabolism in alcoholism. Nutr Res Rev. 2002;1:123–152. - PubMed
-
- Badawy AA-B. Pellagra and alcoholism: a biochemical perspective. Alcohol Alcohol. 2014;49:238–250. - PubMed
-
- Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem. 1995;65:1176–1183. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials