Simvastatin Promotes Cardiac Myocyte Relaxation in Association with Phosphorylation of Troponin I
- PMID: 28469574
- PMCID: PMC5395572
- DOI: 10.3389/fphar.2017.00203
Simvastatin Promotes Cardiac Myocyte Relaxation in Association with Phosphorylation of Troponin I
Abstract
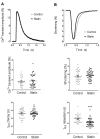
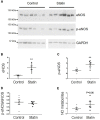
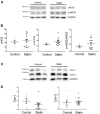
The number of people taking statins is set to increase across the globe due to recent changes in prescription guidelines. For example, half the US population over 40 is now eligible for these drugs, whether they have high serum cholesterol or not. With such development in policy comes a stronger need for understanding statins' myriad of effects. Surprisingly little is known about possible direct actions of statins on cardiac myocytes, although claims of a direct myocardial toxicity have been made. Here, we determine the impact of simvastatin administration (40 mg/kg/day) for 2 weeks in normocholesterolemic rats on cardiac myocyte contractile function and identify an underlying mechanism. Under basal conditions, statin treatment increased the time to half (t0.5) relaxation without any effect on the magnitude of shortening, or the magnitude/kinetics of the [Ca2+]i transient. Enhanced myocyte lusitropy could be explained by a corresponding increase in phosphorylation of troponin I (TnI) at Ser23,24. Statin treatment increased expression of eNOS and Ser1177 phosphorylated eNOS, decreased expression of the NOS-inhibitory proteins caveolins 1 and 3, and increased (P = 0.06) NO metabolites, consistent with enhanced NO production. It is well-established that NO stimulates protein kinase G, one of the effectors of TnI phosphorylation at Ser23,24. Trends for parallel changes in phospho-TnI, phospho-eNOS and caveolin 1 expression were seen in atrial muscle from patients taking statins. Our data are consistent with a mechanism whereby chronic statin treatment enhances TnI phosphorylation and myocyte lusitropy through increased NO bioavailability. We see no evidence of impaired function with statin treatment; the changes we document at the level of the cardiac myocyte should facilitate diastolic filling and cardiac performance.
Keywords: cardiac; caveolin; diastole; lusitropy; nitric oxide; pleiotropy; statin.
Figures






Similar articles
-
Caveolin contributes to the modulation of basal and β-adrenoceptor stimulated function of the adult rat ventricular myocyte by simvastatin: a novel pleiotropic effect.PLoS One. 2014 Sep 11;9(9):e106905. doi: 10.1371/journal.pone.0106905. eCollection 2014. PLoS One. 2014. PMID: 25211146 Free PMC article.
-
Calcitriol modulation of cardiac contractile performance via protein kinase C.J Mol Cell Cardiol. 2006 Aug;41(2):350-9. doi: 10.1016/j.yjmcc.2006.05.019. Epub 2006 Jul 3. J Mol Cell Cardiol. 2006. PMID: 16815434
-
The lack of Troponin I Ser-23/24 phosphorylation is detrimental to in vivo cardiac function and exacerbates cardiac disease.J Mol Cell Cardiol. 2023 Mar;176:84-96. doi: 10.1016/j.yjmcc.2023.01.010. Epub 2023 Jan 29. J Mol Cell Cardiol. 2023. PMID: 36724829 Free PMC article.
-
Suppression of lusitropy as a disease mechanism in cardiomyopathies.Front Cardiovasc Med. 2023 Jan 9;9:1080965. doi: 10.3389/fcvm.2022.1080965. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36698941 Free PMC article. Review.
-
Lipid Screening in Childhood and Adolescence for Detection of Familial Hypercholesterolemia: A Systematic Evidence Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-2. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Aug. Report No.: 14-05204-EF-2. PMID: 27559556 Free Books & Documents. Review.
Cited by
-
Drinking Molecular Hydrogen Water Is Beneficial to Cardiovascular Function in Diet-Induced Obesity Mice.Biology (Basel). 2021 Apr 23;10(5):364. doi: 10.3390/biology10050364. Biology (Basel). 2021. PMID: 33922704 Free PMC article.
-
Simvastatin inhibits proliferation and migration, promotes oxidative stress and ferroptosis in colon cancer.World J Gastrointest Oncol. 2025 May 15;17(5):104522. doi: 10.4251/wjgo.v17.i5.104522. World J Gastrointest Oncol. 2025. PMID: 40487942 Free PMC article.
-
Simvastatin Significantly Reduced Alcohol-Induced Cardiac Damage in Adolescent Mice.Cardiovasc Toxicol. 2024 Jan;24(1):15-26. doi: 10.1007/s12012-023-09821-6. Epub 2024 Jan 23. Cardiovasc Toxicol. 2024. PMID: 38261135 Free PMC article.
References
-
- Bauersachs J., Galuppo P., Fraccarollo D., Christ M., Ertl G. (2001). Improvement of left ventricular remodeling and function by hydroxymethylglutaryl coenzyme a reductase inhibition with cerivastatin in rats with heart failure after myocardial infarction. Circulation 104 982–985. 10.1161/hc3401.095946 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

