Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice
- PMID: 28469619
- PMCID: PMC5395657
- DOI: 10.3389/fimmu.2017.00397
Immune Responses to Broad-Spectrum Antibiotic Treatment and Fecal Microbiota Transplantation in Mice
Abstract
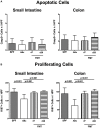
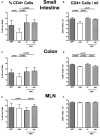
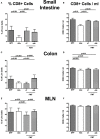
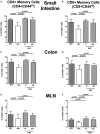
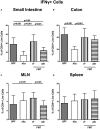
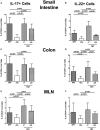
Compelling evidence demonstrates the pivotal role of the commensal intestinal microbiota in host physiology and the detrimental effects of its perturbations following antibiotic treatment. Aim of this study was to investigate the impact of antibiotics induced depletion and subsequent restoration of the intestinal microbiota composition on the murine mucosal and systemic immunity. To address this, conventional C57BL/6j mice were subjected to broad-spectrum antibiotic treatment for 8 weeks. Restoration of the intestinal microbiota by peroral fecal microbiota transplantation (FMT) led to reestablishment of small intestinal CD4+, CD8+, and B220+ as well as of colonic CD4+ cell numbers as early as 7 days post-FMT. However, at d28 following FMT, colonic CD4+ and B220+ cell numbers were comparable to those in secondary abiotic (ABx) mice. Remarkably, CD8+ cell numbers were reduced in the colon upon antibiotic treatment, and FMT was not sufficient to restore this immune cell subset. Furthermore, absence of gut microbial stimuli resulted in decreased percentages of memory/effector T cells, regulatory T cells, and activated dendritic cells in the small intestine, colon, mesenteric lymph nodes (MLN), and spleen. Concurrent antibiotic treatment caused decreased cytokine production (IFN-γ, IL-17, IL-22, and IL-10) of CD4+ cells in respective compartments. These effects were, however, completely restored upon FMT. In summary, broad-spectrum antibiotic treatment resulted in profound local (i.e., small and large intestinal), peripheral (i.e., MLN), and systemic (i.e., splenic) changes in the immune cell repertoire that could, at least in part, be restored upon FMT. Further studies need to unravel the distinct molecular mechanisms underlying microbiota-driven changes in immune homeostasis subsequently providing novel therapeutic or even preventive approaches in human immunopathologies.
Keywords: antibiotics; bacterial recolonization; fecal microbiota transplantation; innate and adaptive immunity; microbiota; mucosal and systemic immune responses; secondary abiotic (gnotobiotic) mice.
Figures







Similar articles
-
Fecal Microbiota Transplantation, Commensal Escherichia coli and Lactobacillus johnsonii Strains Differentially Restore Intestinal and Systemic Adaptive Immune Cell Populations Following Broad-spectrum Antibiotic Treatment.Front Microbiol. 2017 Dec 11;8:2430. doi: 10.3389/fmicb.2017.02430. eCollection 2017. Front Microbiol. 2017. PMID: 29321764 Free PMC article.
-
The Probiotic Compound VSL#3 Modulates Mucosal, Peripheral, and Systemic Immunity Following Murine Broad-Spectrum Antibiotic Treatment.Front Cell Infect Microbiol. 2017 May 5;7:167. doi: 10.3389/fcimb.2017.00167. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28529928 Free PMC article.
-
Intestinal, extra-intestinal and systemic sequelae of Toxoplasma gondii induced acute ileitis in mice harboring a human gut microbiota.PLoS One. 2017 Apr 17;12(4):e0176144. doi: 10.1371/journal.pone.0176144. eCollection 2017. PLoS One. 2017. PMID: 28414794 Free PMC article.
-
Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease.World J Gastroenterol. 2014 Oct 28;20(40):14805-20. doi: 10.3748/wjg.v20.i40.14805. World J Gastroenterol. 2014. PMID: 25356041 Free PMC article. Review.
-
Gut health: The results of microbial and mucosal immune interactions in pigs.Anim Nutr. 2021 Jun;7(2):282-294. doi: 10.1016/j.aninu.2021.01.001. Epub 2021 Mar 25. Anim Nutr. 2021. PMID: 34258416 Free PMC article. Review.
Cited by
-
Peroral Low-Dose Toxoplasma gondii Infection of Human Microbiota-Associated Mice - A Subacute Ileitis Model to Unravel Pathogen-Host Interactions.Eur J Microbiol Immunol (Bp). 2018 Apr 16;8(2):53-61. doi: 10.1556/1886.2018.00005. eCollection 2018 Jun 25. Eur J Microbiol Immunol (Bp). 2018. PMID: 29997912 Free PMC article.
-
The Gut Microbiota of the Greater Horseshoe Bat Confers Rapidly Corresponding Immune Cells in Mice.Animals (Basel). 2025 Feb 26;15(5):685. doi: 10.3390/ani15050685. Animals (Basel). 2025. PMID: 40075967 Free PMC article.
-
Immunological mechanisms of fecal microbiota transplantation in recurrent Clostridioides difficile infection.World J Gastroenterol. 2022 Sep 7;28(33):4762-4772. doi: 10.3748/wjg.v28.i33.4762. World J Gastroenterol. 2022. PMID: 36156924 Free PMC article. Review.
-
Intestinal Microbiota: The Driving Force behind Advances in Cancer Immunotherapy.Cancers (Basel). 2022 Sep 30;14(19):4796. doi: 10.3390/cancers14194796. Cancers (Basel). 2022. PMID: 36230724 Free PMC article. Review.
-
Campylobacter jejuni infection induces acute enterocolitis in IL-10-/- mice pretreated with ampicillin plus sulbactam.Eur J Microbiol Immunol (Bp). 2022 Sep 7;12(3):73-83. doi: 10.1556/1886.2022.00014. Online ahead of print. Eur J Microbiol Immunol (Bp). 2022. PMID: 36069779 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

