Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
- PMID: 28469640
- PMCID: PMC5399703
- DOI: 10.4103/1673-5374.202924
Tissue-type plasminogen activator is a homeostatic regulator of synaptic function in the central nervous system
Abstract
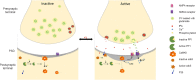
Membrane depolarization induces the release of the serine proteinase tissue-type plasminogen activator (tPA) from the presynaptic terminal of cerebral cortical neurons. Once in the synaptic cleft this tPA promotes the exocytosis and subsequent endocytic retrieval of glutamate-containing synaptic vesicles, and regulates the postsynaptic response to the presynaptic release of glutamate. Indeed, tPA has a bidirectional effect on the composition of the postsynaptic density (PSD) that does not require plasmin generation or the presynaptic release of glutamate, but varies according to the baseline level of neuronal activity. Hence, in inactive neurons tPA induces phosphorylation and accumulation in the PSD of the Ca2+/calmodulin-dependent protein kinase IIα (pCaMKIIα), followed by pCaMKIIα-induced phosphorylation and synaptic recruitment of GluR1-containing α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. In contrast, in active neurons with increased levels of pCaMKIIα in the PSD tPA induces pCaMKIIα and pGluR1 dephosphorylation and their subsequent removal from the PSD. These effects require active synaptic N-methyl-D-aspartate (NMDA) receptors and cyclin-dependent kinase 5 (Cdk5)-induced phosphorylation of the protein phosphatase 1 (PP1) at T320. These data indicate that tPA is a homeostatic regulator of the postsynaptic response of cerebral cortical neurons to the presynaptic release of glutamate via bidirectional regulation of the pCaMKIIα /PP1 switch in the PSD.
Keywords: Ca2+/calmodulin-dependent protein kinase; homeostatic plasticity; plasmin; post-synaptic density; protein phosphatase 1; tissue-type plasminogen activator (tPA).
Conflict of interest statement
Conflicts of interest: None declared.
Figures
Similar articles
-
Tissue-type Plasminogen Activator (tPA) Modulates the Postsynaptic Response of Cerebral Cortical Neurons to the Presynaptic Release of Glutamate.Front Mol Neurosci. 2016 Nov 9;9:121. doi: 10.3389/fnmol.2016.00121. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27881952 Free PMC article.
-
Tissue-type plasminogen activator regulates p35-mediated Cdk5 activation in the postsynaptic terminal.J Cell Sci. 2019 Feb 28;132(5):jcs224196. doi: 10.1242/jcs.224196. J Cell Sci. 2019. PMID: 30709918 Free PMC article.
-
Tissue-type plasminogen activator induces synaptic vesicle endocytosis in cerebral cortical neurons.Neuroscience. 2016 Apr 5;319:69-78. doi: 10.1016/j.neuroscience.2016.01.046. Epub 2016 Jan 26. Neuroscience. 2016. PMID: 26820595 Free PMC article.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Role of the ubiquitin-proteasome system in brain ischemia: friend or foe?Prog Neurobiol. 2014 Jan;112:50-69. doi: 10.1016/j.pneurobio.2013.10.003. Epub 2013 Oct 22. Prog Neurobiol. 2014. PMID: 24157661 Review.
Cited by
-
PAI1 blocks NMDA receptor-mediated effects of tissue-type plasminogen activator on cell signaling and physiology.J Cell Sci. 2018 Jul 26;131(14):jcs217083. doi: 10.1242/jcs.217083. J Cell Sci. 2018. PMID: 29930084 Free PMC article.
-
Tissue-type plasminogen activator induces conditioned receptive field plasticity in the mouse auditory cortex.iScience. 2023 Jan 7;26(2):105947. doi: 10.1016/j.isci.2023.105947. eCollection 2023 Feb 17. iScience. 2023. PMID: 36711245 Free PMC article.
-
Targeting NMDA Receptors at the Neurovascular Unit: Past and Future Treatments for Central Nervous System Diseases.Int J Mol Sci. 2022 Sep 7;23(18):10336. doi: 10.3390/ijms231810336. Int J Mol Sci. 2022. PMID: 36142247 Free PMC article. Review.
-
Intra- and Extracellular Pillars of a Unifying Framework for Homeostatic Plasticity: A Crosstalk Between Metabotropic Receptors and Extracellular Matrix.Front Cell Neurosci. 2019 Nov 19;13:513. doi: 10.3389/fncel.2019.00513. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31803023 Free PMC article. Review.
-
The multifaceted role of plasminogen in inflammation.Cell Signal. 2020 Nov;75:109761. doi: 10.1016/j.cellsig.2020.109761. Epub 2020 Aug 28. Cell Signal. 2020. PMID: 32861744 Free PMC article. Review.
References
-
- Berardi N, Pizzorusso T, Maffei L. Extracellular matrix and visual cortical plasticity: freeing the synapse. Neuron. 2004;44:905–908. - PubMed
-
- Camiolo SM, Thorsen S, Astrup T. Fibrinogenolysis and fibrinolysis with tissue plasminogen activator, urokinase, streptokinase-activated human globulin, and plasmin. Proc Soc Exp Biol Med. 1971;138:277–280. - PubMed
-
- Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78:3114–3124. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

