Stress injuries and autophagy in mouse hippocampus after chronic cold exposure
- PMID: 28469659
- PMCID: PMC5399722
- DOI: 10.4103/1673-5374.202932
Stress injuries and autophagy in mouse hippocampus after chronic cold exposure
Abstract
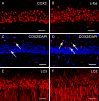
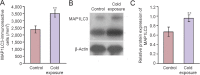
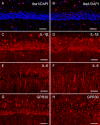
Cold exposure is an external stress factor that causes skin frostbite as well as a variety of diseases. Estrogen might participate in neuroprotection after cold exposure, but its precise mechanism remains unclear. In this study, mice were exposed to 10°C for 7 days and 0-4°C for 30 days to induce a model of chronic cold exposure. Results showed that oxidative stress-related c-fos and cyclooxygenase 2 expressions, MAP1LC3-labeled autophagic cells, Iba1-labeled activated microglia, and interleukin-1β-positive pyramidal cells were increased in the hippocampal CA1 area. Chronic cold exposure markedly elevated the levels of estrogen in the blood and the estrogen receptor, G protein-coupled receptor 30. These results indicate that neuroimmunoreactivity is involved in chronic cold exposure-induced pathological alterations, including oxidative stress, neuronal autophagy, and neuroimmunoreactivity. Moreover, estrogen exerts a neuroprotective effect on cold exposure.
Keywords: autophagy; chronic cold exposure; estrogen; hippocampal CA1 area; microglial cells; nerve regeneration; neural regeneration; neuroimmunoreactivity; oxidative stress.
Conflict of interest statement
Conflicts of interest: None declared.
Figures
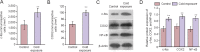

Similar articles
-
Corticosterone Excess-Mediated Mitochondrial Damage Induces Hippocampal Neuronal Autophagy in Mice Following Cold Exposure.Animals (Basel). 2019 Sep 14;9(9):682. doi: 10.3390/ani9090682. Animals (Basel). 2019. PMID: 31540011 Free PMC article.
-
Oxidation Stress-Mediated MAPK Signaling Pathway Activation Induces Neuronal Loss in the CA1 and CA3 Regions of the Hippocampus of Mice Following Chronic Cold Exposure.Brain Sci. 2019 Oct 12;9(10):273. doi: 10.3390/brainsci9100273. Brain Sci. 2019. PMID: 31614701 Free PMC article.
-
The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation.J Neuroinflammation. 2018 Jul 12;15(1):206. doi: 10.1186/s12974-018-1246-x. J Neuroinflammation. 2018. PMID: 30001721 Free PMC article.
-
Effects of chronic cold exposure on murine central nervous system.J Neurosci Res. 2014 Apr;92(4):496-505. doi: 10.1002/jnr.23333. Epub 2014 Jan 28. J Neurosci Res. 2014. PMID: 24474045
-
Neuroprotective effect of estrogen: role of nonsynaptic NR2B-containing NMDA receptors.Brain Res Bull. 2013 Apr;93:27-31. doi: 10.1016/j.brainresbull.2012.10.004. Epub 2012 Oct 17. Brain Res Bull. 2013. PMID: 23085545 Review.
Cited by
-
Dehydrozingerone protects temozolomide-induced cognitive impairment in normal and C6 glioma rats besides enhancing its anticancer potential.3 Biotech. 2020 Oct;10(10):438. doi: 10.1007/s13205-020-02427-7. Epub 2020 Sep 17. 3 Biotech. 2020. PMID: 32995109 Free PMC article.
-
Activation of GPR30 with G1 inhibits oscillatory shear stress-induced adhesion of THP-1 monocytes to HAECs by increasing KLF2.Aging (Albany NY). 2021 Apr 19;13(8):11942-11953. doi: 10.18632/aging.202897. Epub 2021 Apr 19. Aging (Albany NY). 2021. PMID: 33875621 Free PMC article.
-
Mast Cell Activation in Brain Injury, Stress, and Post-traumatic Stress Disorder and Alzheimer's Disease Pathogenesis.Front Neurosci. 2017 Dec 12;11:703. doi: 10.3389/fnins.2017.00703. eCollection 2017. Front Neurosci. 2017. PMID: 29302258 Free PMC article. Review.
-
Effects of hypothermia during propofol anesthesia on learning and memory ability and hippocampal apoptosis in neonatal rats.J Anesth. 2019 Feb;33(1):9-16. doi: 10.1007/s00540-018-2576-7. Epub 2018 Nov 17. J Anesth. 2019. PMID: 30448976
-
How does chronic psychosocial distress induce pain? Focus on neuroinflammation and neuroplasticity changes.Brain Behav Immun Health. 2025 Feb 10;44:100964. doi: 10.1016/j.bbih.2025.100964. eCollection 2025 Mar. Brain Behav Immun Health. 2025. PMID: 40034488 Free PMC article.
References
-
- Bei S, Yifan C, Kunlin J. Estrogen, neuroprotection and neurogenesis after ischemic stroke. Curr Drug Targets. 2012;13:188–198. - PubMed
-
- Bombelli M, Sega R, Facchetti R, Corrao G, Friz HP, Vertemati AM, Sanvito R, Banfi E, Carugo S, Primitz L, Mancia G. Prevalence and clinical significance of a greater ambulatory versus office blood pressure (‘reversed white coat’ condition) in a general population. J Hypertens. 2005;23:513–520. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

