Ibrutinib-Associated Skin Toxicity: A Case of Maculopapular Rash in a 79-Year Old Caucasian Male Patient with Relapsed Waldenstrom's Macroglobulinemia and Review of the Literature
- PMID: 28469834
- PMCID: PMC5402616
- DOI: 10.4081/dr.2017.6976
Ibrutinib-Associated Skin Toxicity: A Case of Maculopapular Rash in a 79-Year Old Caucasian Male Patient with Relapsed Waldenstrom's Macroglobulinemia and Review of the Literature
Abstract
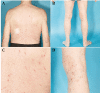
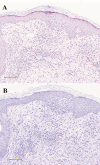
Waldenstrom's macroglobulinamia (WM) is a rare malignant lymphoproliferative disorder, characterized by monoclonal IgM paraproteinemia and neoplastic proliferation of malignant lymphoplasmacytoid cells in the bone marrow. Traditionally, WM has been treated with modalities similar to those used in the management of other indolent lymphomas. Just recently, based on impressive clinical trial results in heavily pretreated WM patients, a new Bruton Tyrosine Kinase-inhibitor, Ibrutinib, has been approved for the treatment of this disorder. As the use of Ibrutinib in WM outside clinical trials is still limited, only few clinical reports illustrating treatment side effects are currently available. Here we review the current literature specific on Ibrutinib-associated rash in hematologic patients, and report on an elderly patient with WM, who developed a red maculopapular non-pruritic rash 12 weeks after starting Ibrutinib therapy. Without modifications of the ongoing Ibrutinib schedule, the rash regressed within two weeks of treatment with topical steroid-containing dermatological compounds.
Keywords: Waldenstrom's macroglobulinamia; adverse effects; ibrutinib; minireview; rash.
Figures
Similar articles
-
Evaluating ibrutinib in the treatment of symptomatic Waldenstrom's macroglobulinemia.J Blood Med. 2019 Aug 27;10:291-300. doi: 10.2147/JBM.S183997. eCollection 2019. J Blood Med. 2019. PMID: 31695539 Free PMC article.
-
The Use of Bruton Tyrosine Kinase Inhibitors in Waldenström's Macroglobulinemia.J Pers Med. 2022 Apr 22;12(5):676. doi: 10.3390/jpm12050676. J Pers Med. 2022. PMID: 35629099 Free PMC article. Review.
-
The Use of Bruton Tyrosine Kinase Inhibitors in Waldenström's Macroglobulinemia.Clin Hematol Int. 2022 May 23;4(1-2):21-29. doi: 10.1007/s44228-022-00007-5. eCollection 2022 Jun. Clin Hematol Int. 2022. PMID: 35950210 Free PMC article. Review.
-
Ibrutinib Efficacy, Safety, and Pharmacokinetics in Chinese Patients with Relapsed or Refractory Waldenström's Macroglobulinemia: A Multicenter, Single-Arm, Phase 4 Study.Adv Ther. 2024 Feb;41(2):672-685. doi: 10.1007/s12325-023-02720-w. Epub 2023 Dec 11. Adv Ther. 2024. PMID: 38079089 Free PMC article. Clinical Trial.
-
Paronychia and Periungual Granulation as a Novel Side Effect of Ibrutinib: A Case Report.Skin Appendage Disord. 2020 Jan;6(1):32-36. doi: 10.1159/000502986. Epub 2019 Oct 8. Skin Appendage Disord. 2020. PMID: 32021859 Free PMC article.
Cited by
-
Ibrutinib-associated pityriasis rosea-like rash.JAAD Case Rep. 2017 Dec 19;4(1):55-57. doi: 10.1016/j.jdcr.2017.06.035. eCollection 2018 Jan. JAAD Case Rep. 2017. PMID: 29387749 Free PMC article. No abstract available.
-
Ibrutinib-Induced Perianal Rash Complicated by Bacterial Infection in a Patient With Chronic Lymphocytic Leukemia: A Case Report and Literature Review.Cureus. 2025 Apr 24;17(4):e82952. doi: 10.7759/cureus.82952. eCollection 2025 Apr. Cureus. 2025. PMID: 40416196 Free PMC article.
-
Macroglobulinemia and Autoinflammatory Disease.Rheumatol Immunol Res. 2021 Dec 31;2(4):227-232. doi: 10.2478/rir-2021-0031. eCollection 2021 Dec. Rheumatol Immunol Res. 2021. PMID: 36467983 Free PMC article. Review.
-
Wells' syndrome possibly caused by hematologic malignancy, influenza vaccination or ibrutinib: A case report.World J Clin Cases. 2022 Oct 26;10(30):10997-11003. doi: 10.12998/wjcc.v10.i30.10997. World J Clin Cases. 2022. PMID: 36338211 Free PMC article.
References
-
- Treon SP, Emmanouilides C, Kimby E, et al. Extended rituximab therapy in Waldenstrom’s macroglobulinemia. Ann Oncol 2005;16:132-8. - PubMed
-
- Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381: 1203-10. - PubMed
-
- Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Primary treatment of Waldenstrom macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol 2007; 25:3344-9. - PubMed
-
- Treon SP, Xu L, Hunter Z. MYD88 Mutations and Response to Ibrutinib in Waldenstrom’s Macroglobulinemia. N Engl J Med 2015;373:584-6. - PubMed
-
- Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenstrom’s macroglobulinemia. N Engl J Med 2012;367:826-33. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

