Developing an ancient epithelial appendage: FGF signalling regulates early tail denticle formation in sharks
- PMID: 28469835
- PMCID: PMC5414203
- DOI: 10.1186/s13227-017-0071-0
Developing an ancient epithelial appendage: FGF signalling regulates early tail denticle formation in sharks
Abstract
Background: Vertebrate epithelial appendages constitute a diverse group of organs that includes integumentary structures such as reptilian scales, avian feathers and mammalian hair. Recent studies have provided new evidence for the homology of integumentary organ development throughout amniotes, despite their disparate final morphologies. These structures develop from conserved molecular signalling centres, known as epithelial placodes. It is not yet certain whether this homology extends beyond the integumentary organs of amniotes, as there is a lack of knowledge regarding their development in basal vertebrates. As the ancient sister lineage of bony vertebrates, extant chondrichthyans are well suited to testing the phylogenetic depth of this homology. Elasmobranchs (sharks, skates and rays) possess hard, mineralised epithelial appendages called odontodes, which include teeth and dermal denticles (placoid scales). Odontodes constitute some of the oldest known vertebrate integumentary appendages, predating the origin of gnathostomes. Here, we used an emerging model shark (Scyliorhinus canicula) to test the hypothesis that denticles are homologous to other placode-derived amniote integumentary organs. To examine the conservation of putative gene regulatory network (GRN) member function, we undertook small molecule inhibition of fibroblast growth factor (FGF) signalling during caudal denticle formation.
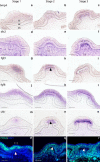
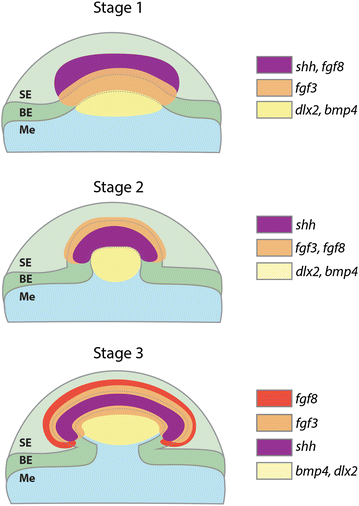
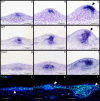
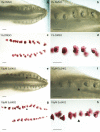
Results: We show that during early caudal denticle morphogenesis, the shark expresses homologues of conserved developmental gene families, known to comprise a core GRN for early placode morphogenesis in amniotes. This includes conserved expression of FGFs, sonic hedgehog (shh) and bone morphogenetic protein 4 (bmp4). Additionally, we reveal that denticle placodes possess columnar epithelial cells with a reduced rate of proliferation, a conserved characteristic of amniote skin appendage development. Small molecule inhibition of FGF signalling revealed placode development is FGF dependent, and inhibiting FGF activity resulted in downregulation of shh and bmp4 expression, consistent with the expectation from comparison to the amniote integumentary appendage GRN.
Conclusion: Overall, these findings suggest the core GRN for building vertebrate integumentary epithelial appendages has been highly conserved over 450 million years. This provides evidence for the continuous, historical homology of epithelial appendage placodes throughout jawed vertebrates, from sharks to mammals. Epithelial placodes constitute the shared foundation upon which diverse vertebrate integumentary organs have evolved.
Keywords: Anatomical placode; Dermal denticle; Epithelial appendage; Homology; Shark.
Figures






Similar articles
-
An ancient Turing-like patterning mechanism regulates skin denticle development in sharks.Sci Adv. 2018 Nov 7;4(11):eaau5484. doi: 10.1126/sciadv.aau5484. eCollection 2018 Nov. Sci Adv. 2018. PMID: 30417097 Free PMC article.
-
Teeth outside the mouth: The evolution and development of shark denticles.Evol Dev. 2023 Jan;25(1):54-72. doi: 10.1111/ede.12427. Epub 2023 Jan 3. Evol Dev. 2023. PMID: 36594351
-
The homology of odontodes in gnathostomes: insights from Dlx gene expression in the dogfish, Scyliorhinus canicula.BMC Evol Biol. 2011 Oct 18;11:307. doi: 10.1186/1471-2148-11-307. BMC Evol Biol. 2011. PMID: 22008058 Free PMC article.
-
Evo Devo of the Vertebrates Integument.J Dev Biol. 2023 Jun 5;11(2):25. doi: 10.3390/jdb11020025. J Dev Biol. 2023. PMID: 37367479 Free PMC article. Review.
-
Origin and evolution of gnathostome dentitions: a question of teeth and pharyngeal denticles in placoderms.Biol Rev Camb Philos Soc. 2005 May;80(2):303-45. doi: 10.1017/s1464793104006682. Biol Rev Camb Philos Soc. 2005. PMID: 15921053 Review.
Cited by
-
An ancient Turing-like patterning mechanism regulates skin denticle development in sharks.Sci Adv. 2018 Nov 7;4(11):eaau5484. doi: 10.1126/sciadv.aau5484. eCollection 2018 Nov. Sci Adv. 2018. PMID: 30417097 Free PMC article.
-
Interference with the retinoic acid signalling pathway inhibits the initiation of teeth and caudal primary scales in the small-spotted catshark Scyliorhinus canicula.PeerJ. 2023 Sep 6;11:e15896. doi: 10.7717/peerj.15896. eCollection 2023. PeerJ. 2023. PMID: 37692112 Free PMC article.
-
Self-organized patterning of crocodile head scales by compressive folding.Nature. 2025 Jan;637(8045):375-383. doi: 10.1038/s41586-024-08268-1. Epub 2024 Dec 11. Nature. 2025. PMID: 39663449 Free PMC article.
-
Leafy and weedy seadragon genomes connect genic and repetitive DNA features to the extravagant biology of syngnathid fishes.Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2119602119. doi: 10.1073/pnas.2119602119. Epub 2022 Jun 22. Proc Natl Acad Sci U S A. 2022. PMID: 35733255 Free PMC article.
-
Exacerbated sonic hedgehog signalling promotes a transition from chemical pre-patterning of chicken reticulate scales to mechanical skin folding.Open Biol. 2025 Apr;15(4):240342. doi: 10.1098/rsob.240342. Epub 2025 Apr 16. Open Biol. 2025. PMID: 40237157 Free PMC article.
References
-
- Sengel P. Pattern formation in skin development. Int J Dev Biol. 1990;34:33–50. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

