Senescence is delayed when ramie (Boehmeria nivea L.) is transformed with the isopentyl transferase (ipt) gene under control of the SAG12 promoter
- PMID: 28469976
- PMCID: PMC5407899
- DOI: 10.1002/2211-5463.12191
Senescence is delayed when ramie (Boehmeria nivea L.) is transformed with the isopentyl transferase (ipt) gene under control of the SAG12 promoter
Abstract
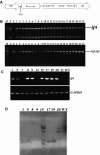
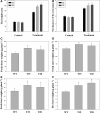
Ramie is an economically important industrial fiber crop widely planted in China, India, and other Southeast Asian and Pacific Rim countries. It plays an important role in China's economy, where ramie farming, industry, and trade provide livelihood support to about five million people. However, poor fiber production resulting from leaf senescence and leaf abscission is a significant problem. In this study, we report the successful production of transgenic ramie plants which delayed leaf senescence and enhanced biomass. Transgenic ramie plants were obtained via transformation with the Agrobacterium tumefaciens strain harboring the binary vector pSG529 containing the isopentyl transferase (ipt) gene under control of the SAG12 promoter (PSAG12-ipt construct). Agrobacterium tumefaciens strain EHA105 was used for the midrib explant transformation. The transformation frequency was 28.29%. Southern blot confirmed the integration of 1-4 copies of the NPTII gene into the ramie genome in the tested lines. At the fiber maturation stage, the transgenic plants had higher photosynthesis rates, chlorophyll content (SPAD values), and stronger resistance to exogenous ethylene compared with wild-type plants.
Keywords: ethylene; ipt; leaf senescence; ramie.
Figures






References
-
- Liu LJ, Lao CY, Zhang N, Chen HQ, Deng G, Zhu C and Peng DX (2013) The effect of new continuous harvest technology of ramie (Boehmeria nivea L. Gaud.) on fiber yield and quality. Ind Crop Prod 44, 677–683.
-
- An X, Wang B, Liu LJ, Jiang H, Chen J, Ye ST, Chen LY, Guo PA, Huang X and Peng DX (2014) Agrobacterium‐mediated genetic transformation and regeneration of transgenic plants using leaf midribs as explants in ramie [Boehmeria nivea (L.) Gaud]. Mol Biol Rep 41, 3257–3269. - PubMed
-
- Miao Y, Laun T, Zimmermann P and Zentgraf U (2004) Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol Biol 55, 853–867. - PubMed
-
- Lim PO, Kim HJ and Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58, 115–136. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

