In Vivo Activation of Duocarmycin-Antibody Conjugates by Near-Infrared Light
- PMID: 28470051
- PMCID: PMC5408340
- DOI: 10.1021/acscentsci.7b00026
In Vivo Activation of Duocarmycin-Antibody Conjugates by Near-Infrared Light
Abstract
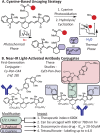
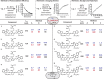
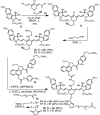
Near-IR photocaging groups based on the heptamethine cyanine scaffold present the opportunity to visualize and then treat diseased tissue with potent bioactive molecules. Here we describe fundamental chemical studies that enable biological validation of this approach. Guided by rational design, including computational analysis, we characterize the impact of structural alterations on the cyanine uncaging reaction. A modest change to the ethylenediamine linker (N,N'-dimethyl to N,N'-diethyl) leads to a bathochromic shift in the absorbance maxima, while decreasing background hydrolysis. Building on these structure-function relationship studies, we prepare antibody conjugates that uncage a derivative of duocarmycin, a potent cytotoxic natural product. The optimal conjugate, CyEt-Pan-Duo, undergoes small molecule release with 780 nm light, exhibits activity in the picomolar range, and demonstrates excellent light-to-dark selectivity. Mouse xenograft studies illustrate that the construct can be imaged in vivo prior to uncaging with an external laser source. Significant reduction in tumor burden is observed following a single dose of conjugate and near-IR light. These studies define key chemical principles that enable the identification of cyanine-based photocages with enhanced properties for in vivo drug delivery.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
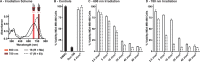

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

