Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma
- PMID: 28470120
- PMCID: PMC6410359
- DOI: 10.1177/0748730417696788
Cell-intrinsic, Bmal1-dependent Circadian Regulation of Temozolomide Sensitivity in Glioblastoma
Abstract
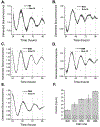
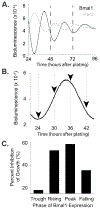
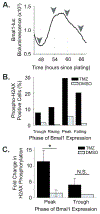
The safety and efficacy of chemotherapeutics can vary as a function of the time of their delivery during the day. This study aimed to improve the treatment of glioblastoma (GBM), the most common brain cancer, by testing whether the efficacy of the DNA alkylator temozolomide (TMZ) varies with the time of its administration. We found cell-intrinsic, daily rhythms in both human and mouse GBM cells. Circadian time of treatment affected TMZ sensitivity of murine GBM tumor cells in vitro. The maximum TMZ-induced DNA damage response, activation of apoptosis, and growth inhibition occurred near the daily peak in expression of the core clock gene Bmal1. Deletion of Bmal1 (Arntl) abolished circadian rhythms in gene expression and TMZ-induced activation of apoptosis and growth inhibition. These data indicate that tumor cell-intrinsic circadian rhythms are common to GBM tumors and can regulate TMZ cytotoxicity. Optimization of GBM treatment by timing TMZ administration to daily rhythms should be evaluated in prospective clinical trials.
Keywords: Bmal1 gene; DNA repair; GBM; H2AX; Period2 gene; astrocytoma; cancer.
Figures

References
-
- Barone A, Sengupta R, Warrington NM, Smith E, Wen PY, Brekken RA, Romagnoli B, Douglas G, Chevalier E, Bauer MP, et al. (2014). Combined VEGF and CXCR4 antagonism targets the GBM stem cell population and synergistically improves survival in an intracranial mouse model of glioblastoma. Oncotarget 5, 9811–9822. - PMC - PubMed
-
- Beale P, Judson I, Moore S, Statkevich P, Marco A, Cutler DL, Reidenberg P, and Brada M (1999). Effect of gastric pH on the relative oral bioavailability and pharmacokinetics of temozolomide. Cancer Chemother Pharmacol 44, 389–394. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

