New perspective on the regionalization of the anterior forebrain in Osteichthyes
- PMID: 28470718
- PMCID: PMC11520958
- DOI: 10.1111/dgd.12348
New perspective on the regionalization of the anterior forebrain in Osteichthyes
Abstract
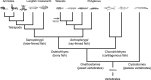
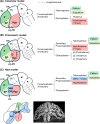
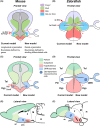
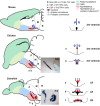
In the current model, the most anterior part of the forebrain (secondary prosencephalon) is subdivided into the telencephalon dorsally and the hypothalamus ventrally. Our recent study identified a new morphogenetic unit named the optic recess region (ORR) between the telencephalon and the hypothalamus. This modification of the forebrain regionalization based on the ventricular organization resolved some previously unexplained inconsistency about regional identification in different vertebrate groups. The ventricular-based comparison also revealed a large diversity within the subregions (notably in the hypothalamus and telencephalon) among different vertebrate groups. In tetrapods there is only one hypothalamic recess, while in teleosts there are two recesses. Most notably, the mammalian and teleost hypothalami are two extreme cases: the former has lost the cerebrospinal fluid-contacting (CSF-c) neurons, while the latter has increased them. Thus, one to one homology of hypothalamic subregions in mammals and teleosts requires careful verification. In the telencephalon, different developmental processes between Sarcopterygii (lobe-finned fish) and Actinopterygii (ray-finned fish) have already been described: the evagination and the eversion. Although pallial homology has been long discussed based on the assumption that the medial-lateral organization of the pallium in Actinopterygii is inverted from that in Sarcopterygii, recent developmental data contradict this assumption. Current models of the brain organization are largely based on a mammalian-centric point of view, but our comparative analyses shed new light on the brain organization of Osteichthyes.
Keywords: evolution; hypothalamus; optic recess region; telencephalon; ventricle.
© 2017 The Authors. Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures

References
-
- Ball, J. N. 1981. Hypothalamic control of the pars distalis in fishes, amphibians, and reptiles. Gen. Comp. Endocrinol. 44, 135–170. - PubMed
-
- Bally‐Cuif, L. & Vernier, P. 2010. Organization and physiology of the zebrafish nervous system. In: Zebrafish (eds Perry S. F., Ekker M., Farrell A. P. & Brauner C. J.), pp. 25–80. Elsevier, Amsterdam.
-
- Bruce, L. L. & Braford, M. R. 2009. Evolution of the limbic system. In: Encyclopedia of Neuroscience (ed. Squire L. R.), Vol. 4, pp. 43–55. Academic Press, Oxford.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

