The role of metals in mammalian olfaction of low molecular weight organosulfur compounds
- PMID: 28471462
- PMCID: PMC5542778
- DOI: 10.1039/c7np00016b
The role of metals in mammalian olfaction of low molecular weight organosulfur compounds
Abstract
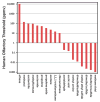
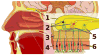
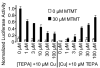
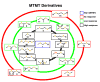
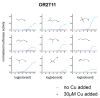
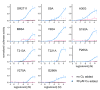
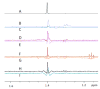
Covering: up to the end of 2017While suggestions concerning the possible role of metals in olfaction and taste date back 50 years, only recently has it been possible to confirm these proposals with experiments involving individual olfactory receptors (ORs). A detailed discussion of recent experimental results demonstrating the key role of metals in enhancing the response of human and other vertebrate ORs to specific odorants is presented against the backdrop of our knowledge of how the sense of smell functions both at the molecular and whole animal levels. This review emphasizes the role of metals in the detection of low molecular weight thiols, sulfides, and other organosulfur compounds, including those found in strong-smelling animal excretions and plant volatiles, and those used in gas odorization. Alternative theories of olfaction are described, with evidence favoring the modified "shape" theory. The use of quantum mechanical/molecular modeling (QM/MM), site-directed mutagenesis and saturation-transfer-difference (STD) NMR is discussed, providing support for biological studies of mouse and human receptors, MOR244-3 and OR OR2T11, respectively. Copper is bound at the active site of MOR244-3 by cysteine and histidine, while cysteine, histidine and methionine are involved with OR2T11. The binding pockets of these two receptors are found in different locations in the three-dimensional seven transmembrane models. Another recently deorphaned human olfactory receptor, OR2M3, highly selective for a thiol from onions, and a broadly-tuned thiol receptor, OR1A1, are also discussed. Other topics covered include the effects of nanoparticles and heavy metal toxicants on vertebrate and fish ORs, intranasal zinc products and the loss of smell (anosmia).
Figures
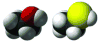
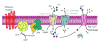


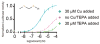
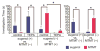

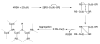







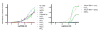





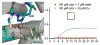




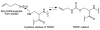
References
-
- Su X, Matsunami H, Zhuang H. In: Bioelectronic Nose. Park TH, editor. Ch. 4. Springer; 2014. pp. 69–81.
-
- Ohloff G, Pickenhagen W, Kraft P. Scent and Chemistry: The Molecular World of Odors. Wiley-VCH; Zurich: 2012.
-
- Andersen KK, Bernstien DT, Caret RL, Romanczyk LJ. Tetrahedron. 1982;38:1965–1970.
- Wood WF, Sollers BG, Dragoo GA, Dragoo JW. J Chem Ecol. 2002;28:1865–1870. - PubMed
-
- Duque C, Bonilla A, Bautista E, Zea S. Biochem Syst Ecol. 2001;29:459–467. - PubMed
- Shirasu M, Fujioka K, Kakishima S, Nagai S, Tomizawa Y, Tsukaya H, Murata J, Manome Y, Touhara K. Biosci Biotechnol Biochem. 2010;74(12):2550–2554. - PubMed
- Stensmyr MC, Urru I, Collu I, Celander M, Hansson BS, Angioy AM. Nature. 2002;420:625–626. - PubMed
-
- Askim JR, Mahmoudi M, Suslick KS. Chem Soc Rev. 2013;42:8649–8682. - PubMed
- Fischer E, Penzoldt F. Justus Liebigs Ann Chem. 1887;239:131–135.
- Kraft P, Mannschreck A. J Chem Educ. 2010;87:598–603.
- Barham P, Skibsted LH, Bredie WLP, Frøst MB, Møller P, Risbo J, Snitkjær P, Mortensen LM. Chem Rev. 2010;110:2313–2365. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

