Circuits and Mechanisms for Surround Modulation in Visual Cortex
- PMID: 28471714
- PMCID: PMC5697758
- DOI: 10.1146/annurev-neuro-072116-031418
Circuits and Mechanisms for Surround Modulation in Visual Cortex
Abstract
Surround modulation (SM) is a fundamental property of sensory neurons in many species and sensory modalities. SM is the ability of stimuli in the surround of a neuron's receptive field (RF) to modulate (typically suppress) the neuron's response to stimuli simultaneously presented inside the RF, a property thought to underlie optimal coding of sensory information and important perceptual functions. Understanding the circuit and mechanisms for SM can reveal fundamental principles of computations in sensory cortices, from mouse to human. Current debate is centered over whether feedforward or intracortical circuits generate SM, and whether this results from increased inhibition or reduced excitation. Here we present a working hypothesis, based on theoretical and experimental evidence, that SM results from feedforward, horizontal, and feedback interactions with local recurrent connections, via synaptic mechanisms involving both increased inhibition and reduced recurrent excitation. In particular, strong and balanced recurrent excitatory and inhibitory circuits play a crucial role in the computation of SM.
Keywords: extrastriate cortex; feedback; horizontal connection; primary visual cortex; recurrent circuits; striate cortex.
Figures
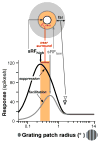
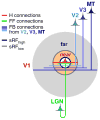



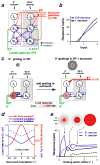
References
-
- Allman J, Miezin F, McGuinness E. Stimulus specific responses from beyond the classical receptive field: neurophysiological mechanisms for local–global comparisons in visual neurons. Annu Rev Neurosci. 1985;8:407–30. - PubMed
-
- Angelucci A, Bressloff PC. The contribution of feedforward, lateral and feedback connections to the classical receptive field center and extra-classical receptive field surround of primate V1 neurons. Prog Brain Res. 2006;154:93–121. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

