Tgm1-like transglutaminases in tilapia (Oreochromis mossambicus)
- PMID: 28472103
- PMCID: PMC5417640
- DOI: 10.1371/journal.pone.0177016
Tgm1-like transglutaminases in tilapia (Oreochromis mossambicus)
Abstract
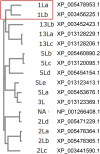
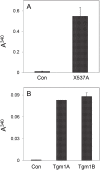
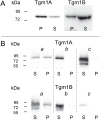
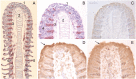
Among the adaptations of aquatic species during evolution of terrestrial tetrapods was the development of an epidermis preventing desiccation. In present day mammals, keratinocytes of the epidermis, using a membrane-bound transglutaminase (Tgm1), accomplish this function by synthesizing a scaffold of cross-linked protein to which a lipid envelope is attached. This study characterizes the abilities of two homologous transglutaminase isozymes in the teleost fish tilapia to form cross-linked protein structures and their expression in certain tissues. Results indicate they are capable of membrane localization and of generating cellular structures resistant to detergent solubilization. They are both expressed in epithelial cells of the lip, buccal cavity and tips of gill filaments. Adaptation of transglutaminase use in evolution of terrestrial keratinocytes evidently involved refinements in tissue expression, access to suitable substrate proteins and activation of cross-linking during terminal differentiation.
Conflict of interest statement
Figures




Similar articles
-
Identification of Chicken Transglutaminase 1 and In Situ Localization of Transglutaminase Activity in Avian Skin and Esophagus.Genes (Basel). 2021 Sep 30;12(10):1565. doi: 10.3390/genes12101565. Genes (Basel). 2021. PMID: 34680960 Free PMC article.
-
Epidermal cell cultures from white and green sturgeon (Acipenser transmontanus and medirostris): Expression of TGM1-like transglutaminases and CYP4501A.PLoS One. 2022 Mar 16;17(3):e0265218. doi: 10.1371/journal.pone.0265218. eCollection 2022. PLoS One. 2022. PMID: 35294467 Free PMC article.
-
Immuno-cross reactivity of transglutaminase and cornification marker proteins in the epidermis of vertebrates suggests common processes of soft cornification across species.J Exp Zool B Mol Dev Evol. 2004 Nov 15;302(6):526-49. doi: 10.1002/jez.b.21016. J Exp Zool B Mol Dev Evol. 2004. PMID: 15468051
-
Bricks and mortar of the epidermal barrier.Exp Mol Med. 1999 Mar 31;31(1):5-19. doi: 10.1038/emm.1999.2. Exp Mol Med. 1999. PMID: 10231017 Review.
-
Analysis of the expression of transglutaminases in the reconstructed human epidermis using a three-dimensional cell culture.Anal Biochem. 2020 Aug 15;603:113606. doi: 10.1016/j.ab.2020.113606. Epub 2020 Jan 28. Anal Biochem. 2020. PMID: 32004543 Review.
Cited by
-
The Evolution of Transglutaminases Underlies the Origin and Loss of Cornified Skin Appendages in Vertebrates.Mol Biol Evol. 2024 Jun 1;41(6):msae100. doi: 10.1093/molbev/msae100. Mol Biol Evol. 2024. PMID: 38781495 Free PMC article.
-
Genomic and physiological mechanisms underlying skin plasticity during water to air transition in an amphibious fish.J Exp Biol. 2021 Jan 26;224(Pt 2):jeb235515. doi: 10.1242/jeb.235515. J Exp Biol. 2021. PMID: 33328287 Free PMC article.
-
Identification of Chicken Transglutaminase 1 and In Situ Localization of Transglutaminase Activity in Avian Skin and Esophagus.Genes (Basel). 2021 Sep 30;12(10):1565. doi: 10.3390/genes12101565. Genes (Basel). 2021. PMID: 34680960 Free PMC article.
-
Epidermal cell cultures from white and green sturgeon (Acipenser transmontanus and medirostris): Expression of TGM1-like transglutaminases and CYP4501A.PLoS One. 2022 Mar 16;17(3):e0265218. doi: 10.1371/journal.pone.0265218. eCollection 2022. PLoS One. 2022. PMID: 35294467 Free PMC article.
-
An epithelial cell culture model for sturgeon integument responds sensitively to 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure.Sci Rep. 2025 Jul 24;15(1):26875. doi: 10.1038/s41598-025-12299-7. Sci Rep. 2025. PMID: 40707633 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

