Constructing the informatics and information technology foundations of a medical device evaluation system: a report from the FDA unique device identifier demonstration
- PMID: 28472359
- PMCID: PMC7647129
- DOI: 10.1093/jamia/ocx041
Constructing the informatics and information technology foundations of a medical device evaluation system: a report from the FDA unique device identifier demonstration
Abstract
Objective: The US Food and Drug Administration (FDA) has recognized the need to improve the tracking of medical device safety and performance, with implementation of Unique Device Identifiers (UDIs) in electronic health information as a key strategy. The FDA funded a demonstration by Mercy Health wherein prototype UDIs were incorporated into its electronic information systems. This report describes the demonstration's informatics architecture.
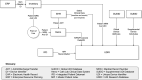
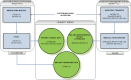
Methods: Prototype UDIs for coronary stents were created and implemented across a series of information systems, resulting in UDI-associated data flow from manufacture through point of use to long-term follow-up, with barcode scanning linking clinical data with UDI-associated device attributes. A reference database containing device attributes and the UDI Research and Surveillance Database (UDIR) containing the linked clinical and device information were created, enabling longitudinal assessment of device performance. The demonstration included many stakeholders: multiple Mercy departments, manufacturers, health system partners, the FDA, professional societies, the National Cardiovascular Data Registry, and information system vendors.
Results: The resulting system of systems is described in detail, including entities, functions, linkage between the UDIR and proprietary systems using UDIs as the index key, data flow, roles and responsibilities of actors, and the UDIR data model.
Conclusion: The demonstration provided proof of concept that UDIs can be incorporated into provider and enterprise electronic information systems and used as the index key to combine device and clinical data in a database useful for device evaluation. Keys to success and challenges to achieving this goal were identified. Fundamental informatics principles were central to accomplishing the system of systems model.
Keywords: device research; medical devices; safety surveillance; system of systems.
© The Author 2017. Published by Oxford University Press on behalf of the American Medical Informatics Association. All rights reserved. For Permissions, please email: journals.permissions@oup.com
Figures

References
-
- Strengthening Our National System for Medical Device Postmarket Surveillance. 2012; http://www.fda.gov/downloads/AboutFDA/CentersOffices/OfficeofMedicalProd.... Accessed April 20, 2017.
-
- Strengthening Our National System for Medical Device Postmarket Surveillance: Update and Next Steps. 2013. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTob.... Accessed July 27, 2015.
-
- Normand SL, Hatfield L, Drozda J, et al. . Postmarket surveillance for medical devices: America’s new strategy. BMJ. 2012;345:e6848. - PubMed
-
- Gross TP, Crowley J. Unique device identification in the service of public health. N Engl J Med. 2012;36717:1583–85. - PubMed
-
- US Food and Drug Administration. Global UDI Database (GUDID). http://www.fda.gov/medicaldevices/deviceregulationandguidance/uniquedevi.... Accessed March 29, 2016.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

