Energy Metabolism of the Osteoblast: Implications for Osteoporosis
- PMID: 28472361
- PMCID: PMC5460680
- DOI: 10.1210/er.2017-00064
Energy Metabolism of the Osteoblast: Implications for Osteoporosis
Abstract
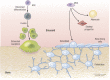
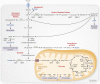
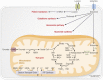
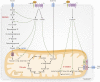
Osteoblasts, the bone-forming cells of the remodeling unit, are essential for growth and maintenance of the skeleton. Clinical disorders of substrate availability (e.g., diabetes mellitus, anorexia nervosa, and aging) cause osteoblast dysfunction, ultimately leading to skeletal fragility and osteoporotic fractures. Conversely, anabolic treatments for osteoporosis enhance the work of the osteoblast by altering osteoblast metabolism. Emerging evidence supports glycolysis as the major metabolic pathway to meet ATP demand during osteoblast differentiation. Glut1 and Glut3 are the principal transporters of glucose in osteoblasts, although Glut4 has also been implicated. Wnt signaling induces osteoblast differentiation and activates glycolysis through mammalian target of rapamycin, whereas parathyroid hormone stimulates glycolysis through induction of insulin-like growth factor-I. Glutamine is an alternate fuel source for osteogenesis via the tricarboxylic acid cycle, and fatty acids can be metabolized to generate ATP via oxidative phosphorylation although temporal specificity has not been established. More studies with new model systems are needed to fully understand how the osteoblast utilizes fuel substrates in health and disease and how that impacts metabolic bone diseases.
Copyright © 2017 Endocrine Society.
Figures
References
-
- Borle AB, Nichols N, Nichols G Jr. Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:1206–1210. - PubMed
-
- Cohn DV, Forscher BK. Aerobic metabolism of glucose by bone. J Biol Chem. 1962;237:615–618. - PubMed
-
- Peck WA, Birge SJ Jr, Fedak SA. Bone Cells: Biochemical and Biological Studies after Enzymatic Isolation. Science. 1964;146(3650):1476–1477. - PubMed
-
- Peck WA, Birge SJ Jr, Brandt J. Collagen synthesis by isolated bone cells: stimulation by ascorbic acid in vitro. Biochim Biophys Acta. 1967;142(2):512–525. - PubMed
-
- Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987;122(1):49–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

