Multicenter phase II study of temozolomide and myeloablative chemotherapy with autologous stem cell transplant for newly diagnosed anaplastic oligodendroglioma
- PMID: 28472509
- PMCID: PMC5596171
- DOI: 10.1093/neuonc/nox086
Multicenter phase II study of temozolomide and myeloablative chemotherapy with autologous stem cell transplant for newly diagnosed anaplastic oligodendroglioma
Abstract
Background: Anaplastic oligodendroglioma (AO) and anaplastic oligoastrocytoma (AOA) are chemotherapy-sensitive tumors with prolonged survival after radiochemotherapy. We report a prospective trial using induction temozolomide (TMZ) followed by myeloablative high-dose chemotherapy (HDC) with autologous stem-cell transplant (ASCT) as a potential strategy to defer radiotherapy.
Methods: Patients with AO/AOA received 6 cycles of TMZ (200 mg/m2 × 5/28 day). Responding patients were eligible for HDC (thiotepa 250 mg/m2/day × 3 days, then busulfan 3.2 mg/kg/day × 3 days), followed by ASCT. Genomic characterization was performed using next-generation sequencing.
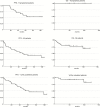
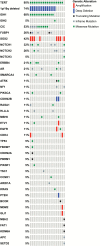
Results: Forty-one patients were enrolled; 85% had 1p/19q codeleted tumors. After induction, 26 patients were eligible for HDC-ASCT and 21 agreed to proceed. There were no unexpected adverse events or toxic deaths. After median follow-up of 66 months, 2-year progression-free survival (PFS) for transplanted patients was 86%, 5-year PFS 60%, and no patient has died. Among all 1p/19q codeleted patients (N = 33), 5-year PFS was 50% and 5-year overall survival (OS) 93%, with median time to radiotherapy not reached. Next-generation sequencing disclosed typical oligodendroglioma-related mutations, including IDH1, TERT, CIC, and FUBP1 mutations in 1p/19q codeleted patients, and glioblastoma-like signatures in 1p/19q intact patients. Aside from IDH1, potentially oncogenic/actionable mutations were variable, depicting wide molecular heterogeneity within oligodendroglial tumors.
Conclusions: TMZ followed by HDC-ASCT can be safely administered to patients with newly diagnosed 1p/19q codeleted AO. This strategy was associated with promising PFS and OS, suggesting that a chemotherapy-based approach may delay the need for radiotherapy and radiation-related toxicities. Raw data for further genomic and meta-analyses are publicly available at http://cbioportal.org/study?id=odg_msk_2017, accessed 6 January 2017.
Clinicaltrials.gov registry: NCT00588523.
Keywords: 1p/19q codeletion; anaplastic oligodendroglioma; autologous stem cell transplant; temozolomide.
© The Author(s) 2017. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com
Figures
Comment in
-
Does intensification of chemotherapy in anaplastic oligodendrogliomas still have a role?Neuro Oncol. 2017 Oct 1;19(10):1292-1293. doi: 10.1093/neuonc/nox146. Neuro Oncol. 2017. PMID: 28922863 Free PMC article. No abstract available.
Similar articles
-
Phase II trial of pre-irradiation and concurrent temozolomide in patients with newly diagnosed anaplastic oligodendrogliomas and mixed anaplastic oligoastrocytomas: long term results of RTOG BR0131.J Neurooncol. 2015 Sep;124(3):413-20. doi: 10.1007/s11060-015-1845-7. Epub 2015 Jun 19. J Neurooncol. 2015. PMID: 26088460 Free PMC article. Clinical Trial.
-
Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide.Int J Radiat Oncol Biol Phys. 2015 Feb 1;91(2):268-76. doi: 10.1016/j.ijrobp.2014.10.027. Int J Radiat Oncol Biol Phys. 2015. PMID: 25636755
-
Efficacy and patient-reported outcomes with dose-intense temozolomide in patients with newly diagnosed pure and mixed anaplastic oligodendroglioma: a phase II multicenter study.J Neurooncol. 2015 Mar;122(1):111-9. doi: 10.1007/s11060-014-1684-y. Epub 2014 Dec 23. J Neurooncol. 2015. PMID: 25534576 Clinical Trial.
-
Complete durable response of a pediatric anaplastic oligodendroglioma to temozolomide alone: Case report and review of literature.Pediatr Blood Cancer. 2017 Dec;64(12):10.1002/pbc.26708. doi: 10.1002/pbc.26708. Epub 2017 Jul 11. Pediatr Blood Cancer. 2017. PMID: 28696020 Free PMC article. Review.
-
Low-grade and anaplastic oligodendroglioma.Handb Clin Neurol. 2016;134:361-80. doi: 10.1016/B978-0-12-802997-8.00022-0. Handb Clin Neurol. 2016. PMID: 26948366 Review.
Cited by
-
Radiomics-Based Machine Learning Technology Enables Better Differentiation Between Glioblastoma and Anaplastic Oligodendroglioma.Front Oncol. 2019 Nov 5;9:1164. doi: 10.3389/fonc.2019.01164. eCollection 2019. Front Oncol. 2019. PMID: 31750250 Free PMC article.
-
Neomorphic PDGFRA extracellular domain driver mutations are resistant to PDGFRA targeted therapies.Nat Commun. 2018 Nov 2;9(1):4583. doi: 10.1038/s41467-018-06949-w. Nat Commun. 2018. PMID: 30389923 Free PMC article.
-
Mutant Isocitrate Dehydrogenase 1 Disrupts PKM2-β-Catenin-BRG1 Transcriptional Network-Driven CD47 Expression.Mol Cell Biol. 2018 Apr 16;38(9):e00001-18. doi: 10.1128/MCB.00001-18. Print 2018 May 1. Mol Cell Biol. 2018. PMID: 29463646 Free PMC article.
-
Early results from the CODEL trial for anaplastic oligodendrogliomas: is temozolomide futile?Neuro Oncol. 2021 Mar 25;23(3):347-349. doi: 10.1093/neuonc/noab006. Neuro Oncol. 2021. PMID: 33560350 Free PMC article. No abstract available.
-
TNPO1-Mediated Nuclear Import of FUBP1 Contributes to Tumor Immune Evasion by Increasing NRP1 Expression in Cervical Cancer.J Immunol Res. 2021 Apr 24;2021:9994004. doi: 10.1155/2021/9994004. eCollection 2021. J Immunol Res. 2021. PMID: 33987449 Free PMC article.
References
-
- Macdonald DR, Gaspar LE, Cairncross JG. Successful chemotherapy for newly diagnosed aggressive oligodendroglioma. Ann Neurol. 1990;27(5):573–574. - PubMed
-
- Cairncross JG, Macdonald DR. Chemotherapy for oligodendroglioma. Progress report. Arch Neurol. 1991;48(2):225–227. - PubMed
-
- Walker MD, Alexander E Jr, Hunt WE et al. . Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49(3):333–343. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

