Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions
- PMID: 28472674
- PMCID: PMC5647777
- DOI: 10.1016/j.fct.2017.04.045
Saccharin induced liver inflammation in mice by altering the gut microbiota and its metabolic functions
Abstract
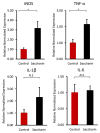
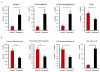
Maintaining the balance of the gut microbiota and its metabolic functions is vital for human health, however, this balance can be disrupted by various external factors including food additives. A range of food and beverages are sweetened by saccharin, which is generally considered to be safe despite controversial debates. However, recent studies indicated that saccharin perturbed the gut microbiota. Inflammation is frequently associated with disruptions of the gut microbiota. The aim of this study is to investigate the relationship between host inflammation and perturbed gut microbiome by saccharin. C57BL/6J male mice were treated with saccharin in drinking water for six months. Q-PCR was used to detect inflammatory markers in mouse liver, while 16S rRNA gene sequencing and metabolomics were used to reveal changes of the gut microbiota and its metabolomic profiles. Elevated expression of pro-inflammatory iNOS and TNF-α in liver indicated that saccharin induced inflammation in mice. The altered gut bacterial genera, enriched orthologs of pathogen-associated molecular patterns, such as LPS and bacterial toxins, in concert with increased pro-inflammatory metabolites suggested that the saccharin-induced liver inflammation could be associated with the perturbation of the gut microbiota and its metabolic functions.
Keywords: Artificial sweetener; Gut microbiota; Inflammation; Metabolite; Saccharin.
Copyright © 2017. Published by Elsevier Ltd.
Figures




References
-
- Atkinson C, Frankenfeld Cara L, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Experimental biology and medicine. 2005;230(3):155–170. - PubMed
-
- Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, … Gillevet PM. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303(6):G675–685. doi: 10.1152/ajpgi.00152.2012. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

