Combined Anti-VEGF and Anti-CTLA-4 Therapy Elicits Humoral Immunity to Galectin-1 Which Is Associated with Favorable Clinical Outcomes
- PMID: 28473314
- PMCID: PMC5509159
- DOI: 10.1158/2326-6066.CIR-16-0385
Combined Anti-VEGF and Anti-CTLA-4 Therapy Elicits Humoral Immunity to Galectin-1 Which Is Associated with Favorable Clinical Outcomes
Abstract
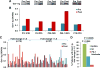
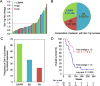
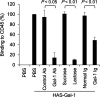
The combination of anti-VEGF blockade (bevacizumab) with immune checkpoint anti-CTLA-4 blockade (ipilimumab) in a phase I study showed tumor endothelial activation and immune cell infiltration that were associated with favorable clinical outcomes in patients with metastatic melanoma. To identify potential immune targets responsible for these observations, posttreatment plasma from long-term responding patients were used to screen human protein arrays. We reported that ipilimumab plus bevacizumab therapy elicited humoral immune responses to galectin-1 (Gal-1), which exhibits protumor, proangiogenesis, and immunosuppressive activities in 37.2% of treated patients. Gal-1 antibodies purified from posttreatment plasma suppressed the binding of Gal-1 to CD45, a T-cell surface receptor that transduces apoptotic signals upon binding to extracellular Gal-1. Antibody responses to Gal-1 were found more frequently in the group of patients with therapeutic responses and correlated with improved overall survival. In contrast, another subgroup of treated patients had increased circulating Gal-1 protein instead, and they had reduced overall survival. Our findings suggest that humoral immunity to Gal-1 may contribute to the efficacy of anti-VEGF and anti-CTLA-4 combination therapy. Gal-1 may offer an additional therapeutic target linking anti-angiogenesis and immune checkpoint blockade. Cancer Immunol Res; 5(6); 446-54. ©2017 AACR.
©2017 American Association for Cancer Research.
Figures

Similar articles
-
VEGF Neutralization Plus CTLA-4 Blockade Alters Soluble and Cellular Factors Associated with Enhancing Lymphocyte Infiltration and Humoral Recognition in Melanoma.Cancer Immunol Res. 2016 Oct;4(10):858-868. doi: 10.1158/2326-6066.CIR-16-0084. Epub 2016 Aug 22. Cancer Immunol Res. 2016. PMID: 27549123 Free PMC article. Clinical Trial.
-
Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy.Cancer Immunol Res. 2017 Jan;5(1):17-28. doi: 10.1158/2326-6066.CIR-16-0206. Epub 2016 Dec 21. Cancer Immunol Res. 2017. PMID: 28003187 Free PMC article.
-
Anti-CTLA-4 based therapy elicits humoral immunity to galectin-3 in patients with metastatic melanoma.Oncoimmunology. 2018 Mar 13;7(7):e1440930. doi: 10.1080/2162402X.2018.1440930. eCollection 2018. Oncoimmunology. 2018. PMID: 29900046 Free PMC article.
-
Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1.J Immunotoxicol. 2012 Jul-Sep;9(3):241-7. doi: 10.3109/1547691X.2012.678021. Epub 2012 Apr 23. J Immunotoxicol. 2012. PMID: 22524673 Review.
-
Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions.Cancer Treat Rev. 2017 Jun;57:36-49. doi: 10.1016/j.ctrv.2017.05.003. Epub 2017 May 18. Cancer Treat Rev. 2017. PMID: 28550712 Review.
Cited by
-
Combinatorial therapy of immune checkpoint and cancer pathways provides a novel perspective on ovarian cancer treatment.Oncol Lett. 2019 Mar;17(3):2583-2591. doi: 10.3892/ol.2019.9902. Epub 2019 Jan 8. Oncol Lett. 2019. PMID: 30854033 Free PMC article. Review.
-
Nano-immunotherapy for each stage of cancer cellular immunity: which, why, and what?Theranostics. 2021 Jun 1;11(15):7471-7487. doi: 10.7150/thno.59953. eCollection 2021. Theranostics. 2021. PMID: 34158861 Free PMC article. Review.
-
Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment.Mol Cancer. 2019 Mar 30;18(1):60. doi: 10.1186/s12943-019-0974-6. Mol Cancer. 2019. PMID: 30925919 Free PMC article. Review.
-
B Cells in Patients With Melanoma: Implications for Treatment With Checkpoint Inhibitor Antibodies.Front Immunol. 2021 Jan 25;11:622442. doi: 10.3389/fimmu.2020.622442. eCollection 2020. Front Immunol. 2021. PMID: 33569063 Free PMC article. Review.
-
Old Player-New Tricks: Non Angiogenic Effects of the VEGF/VEGFR Pathway in Cancer.Cancers (Basel). 2020 Oct 27;12(11):3145. doi: 10.3390/cancers12113145. Cancers (Basel). 2020. PMID: 33121034 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

