4-1BB-Enhanced Expansion of CD8+ TIL from Triple-Negative Breast Cancer Unveils Mutation-Specific CD8+ T Cells
- PMID: 28473315
- PMCID: PMC5525328
- DOI: 10.1158/2326-6066.CIR-16-0364
4-1BB-Enhanced Expansion of CD8+ TIL from Triple-Negative Breast Cancer Unveils Mutation-Specific CD8+ T Cells
Abstract
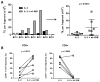
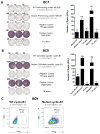
Triple-negative breast cancer (TNBC) highly infiltrated with CD8+ tumor-infiltrating lymphocytes (TIL) has been associated with improved prognosis. This observation led us to hypothesize that CD8+ TIL could be utilized in autologous adoptive cell therapy for TNBC, although this concept has proven to be challenging, given the difficulty in expanding CD8+ TILs in solid cancers other than in melanoma. To overcome this obstacle, we used an agonistic antibody (urelumab) to a TNFR family member, 4-1BB/CD137, which is expressed by recently activated CD8+ T cells. This approach was first utilized in melanoma and, in this study, led to advantageous growth of TILs for the majority of TNBC tumors tested. The agonistic antibody was only added in the initial setting of the culture and yet favored the propagation of CD8+ TILs from TNBC tumors. These expanded CD8+ TILs were capable of cytotoxic functions and were successfully utilized to demonstrate the presence of immunogenic mutations in autologous TNBC tumor tissue without recognition of the wild-type counterpart. Our findings open the way for a successful adoptive immunotherapy for TNBC. Cancer Immunol Res; 5(6); 439-45. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures

References
-
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. - PubMed
-
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:860–7. - PubMed
-
- Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–55. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

