Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis
- PMID: 28473423
- PMCID: PMC5561378
- DOI: 10.1136/annrheumdis-2016-210724
Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis
Abstract
Objectives: To assess the efficacy and safety of abatacept, a selective T-cell costimulation modulator, in a phase III study in psoriatic arthritis (PsA).
Methods: This study randomised patients (1:1) with active PsA (~60% with prior exposure to a tumour necrosis factor inhibitor) to blinded weekly subcutaneous abatacept 125 mg (n=213) or placebo (n=211) for 24 weeks, followed by open-label subcutaneous abatacept. Patients without ≥20% improvement in joint counts at week 16 were switched to open-label abatacept. The primary end point was the proportion of patients with ≥20% improvement in the American College of Rheumatology (ACR20) criteria at week 24.
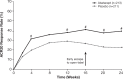
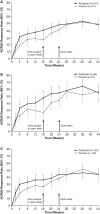
Results: Abatacept significantly increased ACR20 response versus placebo at week 24 (39.4% vs 22.3%; p<0.001). Although abatacept numerically increased Health Assessment Questionnaire-Disability Index response rates (reduction from baseline ≥0.35) at week 24, this was not statistically significant (31.0% vs 23.7%; p=0.097). The benefits of abatacept were seen in ACR20 responses regardless of tumour necrosis factor inhibitor exposure and in other musculoskeletal manifestations, but significance could not be attributed due to ranking below Health Assessment Questionnaire-Disability Index response in hierarchical testing. However, the benefit on psoriasis lesions was modest. Efficacy was maintained or improved up to week 52. Abatacept was well tolerated with no new safety signals.
Conclusions: Abatacept treatment of PsA in this phase III study achieved its primary end point, ACR20 response, showed beneficial trends overall in musculoskeletal manifestations and was well tolerated. There was only a modest impact on psoriasis lesions.
Trial registration number: ClinicalTrials.gov number, NCT01860976 (funded by Bristol-Myers Squibb).
Keywords: DMARDs (biologic); Psoriatic arthritis; T cells; Treatment.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: PM reports receiving consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Corrona, Crescendo, Demira, Janssen, Lilly, Merck, Novartis, Pfizer, Sun, UCB and Zynerba; and speaker fees from AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Novartis, Pfizer and UCB. ABG reports receiving consulting fees from Abbott Laboratories (AbbVie), Actelion, Akros, Amgen, Astellas, Baxalta, Beiersdorf, Bristol-Myers Squibb, Canfite, Catabasis, Celgene, Centocor (Janssen), Coronado, CSL Behring Biotherapies for Life, Dermipsor, Genentech, GlaxoSmithKline, Incyte, Karyopharm, Kineta One, KPI Therapeutics, Lilly, Meiji Seika Pharma, Mitsubishi Tanabe Pharma Development America, Novartis, Novo Nordisk, Pfizer, Takeda, TEVA, UCB, Vertex and Xenoport; and research grants (paid to Tufts Medical Center) from Abbott Laboratories (AbbVie), Amgen, Baxalta, Celgene, Centocor (Janssen), Dermira, Levia, Lilly, Merck, Novartis, Pfizer and Xenoport. DvdH reports receiving consultancy fees from Bristol-Myers Squibb and also consultancy fees from AbbVie, Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Celgene, Daiichi, Eli Lilly, Galapagos, Gilead, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi and UCB (outside the submitted work). OF reports receiving grant support from AbbVie, Bristol-Myers Squibb and Pfizer; and speaker fees from Celgene, Janssen, Novartis and UCB. AJ is an employee of Bristol-Myers Squibb and reports holding stock in Bristol-Myers Squibb. MN is an employee of Bristol-Myers Squibb. SB is an employee of Bristol-Myers Squibb and reports holding stock in Bristol-Myers Squibb. DG reports receiving grant support from Bristol-Myers Squibb to participate in this study; in addition, she reports receiving grant support and fees from AbbVie, Amgen, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB.
Figures
Similar articles
-
Improved patient-reported outcomes in patients with psoriatic arthritis treated with abatacept: results from a phase 3 trial.Arthritis Res Ther. 2018 Dec 6;20(1):269. doi: 10.1186/s13075-018-1769-7. Arthritis Res Ther. 2018. PMID: 30522501 Free PMC article. Clinical Trial.
-
Abatacept in the treatment of patients with psoriatic arthritis: results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial.Arthritis Rheum. 2011 Apr;63(4):939-48. doi: 10.1002/art.30176. Arthritis Rheum. 2011. PMID: 21128258 Clinical Trial.
-
Poor prognostic factors in predicting abatacept response in a phase III randomized controlled trial in psoriatic arthritis.Rheumatol Int. 2020 Jul;40(7):1021-1028. doi: 10.1007/s00296-020-04564-x. Epub 2020 Apr 30. Rheumatol Int. 2020. PMID: 32356115 Free PMC article. Clinical Trial.
-
Etanercept: an updated review of its use in rheumatoid arthritis, psoriatic arthritis and juvenile rheumatoid arthritis.Drugs. 2002;62(17):2493-537. doi: 10.2165/00003495-200262170-00013. Drugs. 2002. PMID: 12421111 Review.
-
Golimumab for the treatment of psoriatic arthritis: a NICE single technology appraisal.Pharmacoeconomics. 2012 Apr;30(4):257-70. doi: 10.2165/11595920-000000000-00000. Pharmacoeconomics. 2012. PMID: 22283690 Review.
Cited by
-
Network Meta-Analysis of Tofacitinib, Biologic Disease-Modifying Antirheumatic Drugs, and Apremilast for the Treatment of Psoriatic Arthritis.Curr Ther Res Clin Exp. 2020 Aug 12;93:100601. doi: 10.1016/j.curtheres.2020.100601. eCollection 2020. Curr Ther Res Clin Exp. 2020. PMID: 32983284 Free PMC article.
-
Consensus statements for pharmacological management, monitoring of therapies, and comorbidity management of psoriatic arthritis in the United Arab Emirates.Int J Rheum Dis. 2022 Oct;25(10):1107-1122. doi: 10.1111/1756-185X.14406. Epub 2022 Aug 2. Int J Rheum Dis. 2022. PMID: 35916205 Free PMC article. Review.
-
Functional Impact of Risk Gene Variants on the Autoimmune Responses in Type 1 Diabetes.Front Immunol. 2022 May 4;13:886736. doi: 10.3389/fimmu.2022.886736. eCollection 2022. Front Immunol. 2022. PMID: 35603161 Free PMC article. Review.
-
Current treatment options for psoriatic arthritis: spotlight on abatacept.Ther Clin Risk Manag. 2018 Jun 6;14:1053-1059. doi: 10.2147/TCRM.S148586. eCollection 2018. Ther Clin Risk Manag. 2018. PMID: 29922065 Free PMC article. Review.
-
Belatacept and regulatory T cells in transplantation: synergistic strategies for immune tolerance and graft survival.Clin Transplant Res. 2024 Dec 31;38(4):326-340. doi: 10.4285/ctr.24.0057. Epub 2024 Dec 18. Clin Transplant Res. 2024. PMID: 39690903 Free PMC article. Review.
References
-
- Ungprasert P, Thongprayoon C, Davis JM. Indirect comparisons of the efficacy of subsequent biological agents in patients with psoriatic arthritis with an inadequate response to tumor necrosis factor inhibitors: a meta-analysis. Clin Rheumatol 2016;35:1795–803.10.1007/s10067-016-3204-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

