Hepatic protein phosphatase 1 regulatory subunit 3B (Ppp1r3b) promotes hepatic glycogen synthesis and thereby regulates fasting energy homeostasis
- PMID: 28473467
- PMCID: PMC5481556
- DOI: 10.1074/jbc.M116.766329
Hepatic protein phosphatase 1 regulatory subunit 3B (Ppp1r3b) promotes hepatic glycogen synthesis and thereby regulates fasting energy homeostasis
Abstract
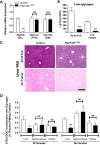
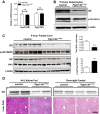
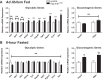
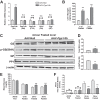
Maintenance of whole-body glucose homeostasis is critical to glycemic function. Genetic variants mapping to chromosome 8p23.1 in genome-wide association studies have been linked to glycemic traits in humans. The gene of known function closest to the mapped region, PPP1R3B (protein phosphatase 1 regulatory subunit 3B), encodes a protein (GL) that regulates glycogen metabolism in the liver. We therefore sought to test the hypothesis that hepatic PPP1R3B is associated with glycemic traits. We generated mice with either liver-specific deletion (Ppp1r3bΔhep ) or liver-specific overexpression of Ppp1r3b The Ppp1r3b deletion significantly reduced glycogen synthase protein abundance, and the remaining protein was predominantly phosphorylated and inactive. As a consequence, glucose incorporation into hepatic glycogen was significantly impaired, total hepatic glycogen content was substantially decreased, and mice lacking hepatic Ppp1r3b had lower fasting plasma glucose than controls. The concomitant loss of liver glycogen impaired whole-body glucose homeostasis and increased hepatic expression of glycolytic enzymes in Ppp1r3bΔhep mice relative to controls in the postprandial state. Eight hours of fasting significantly increased the expression of two critical gluconeogenic enzymes, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase, above the levels in control livers. Conversely, the liver-specific overexpression of Ppp1r3b enhanced hepatic glycogen storage above that of controls and, as a result, delayed the onset of fasting-induced hypoglycemia. Moreover, mice overexpressing hepatic Ppp1r3b upon long-term fasting (12-36 h) were protected from blood ketone-body accumulation, unlike control and Ppp1r3bΔhep mice. These findings indicate a major role for Ppp1r3b in regulating hepatic glycogen stores and whole-body glucose/energy homeostasis.
Keywords: Alb-Cre; Ppp1r3b; gluconeogenesis; glycogen; glycogen storage disease; glycogen synthase; glycolysis; liver.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
Fasting-induced protein phosphatase 1 regulatory subunit contributes to postprandial blood glucose homeostasis via regulation of hepatic glycogenesis.Diabetes. 2011 May;60(5):1435-45. doi: 10.2337/db10-1663. Epub 2011 Apr 6. Diabetes. 2011. PMID: 21471512 Free PMC article.
-
Ppp1r3b is a metabolic switch that shifts hepatic energy storage from lipid to glycogen.Sci Adv. 2025 May 16;11(20):eado3440. doi: 10.1126/sciadv.ado3440. Epub 2025 May 16. Sci Adv. 2025. PMID: 40378221 Free PMC article.
-
Decreased fasting blood glucose is associated with impaired hepatic glucose production in thyroid-stimulating hormone receptor knockout mice.Endocr J. 2013;60(8):941-50. doi: 10.1507/endocrj.ej12-0462. Epub 2013 May 10. Endocr J. 2013. PMID: 23665701
-
[Glycemic regulation. Application to the diagnosis of hypoglycemia in children (excluding neonatal hypoglycemia)].Ann Pediatr (Paris). 1978 Mar-Apr;25(3-4):153-60. Ann Pediatr (Paris). 1978. PMID: 16114320 Review. French. No abstract available.
-
Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis.Diabetes Metab Res Rev. 2001 Jul-Aug;17(4):250-72. doi: 10.1002/dmrr.217. Diabetes Metab Res Rev. 2001. PMID: 11544610 Review.
Cited by
-
Increased frequency of rare missense PPP1R3B variants among Danish patients with type 2 diabetes.PLoS One. 2019 Jan 10;14(1):e0210114. doi: 10.1371/journal.pone.0210114. eCollection 2019. PLoS One. 2019. PMID: 30629617 Free PMC article.
-
Maintenance of liver glycogen during long-term fasting preserves energy state in mice.FEBS Lett. 2020 Jun;594(11):1698-1710. doi: 10.1002/1873-3468.13770. Epub 2020 Mar 21. FEBS Lett. 2020. PMID: 32159852 Free PMC article.
-
An epidemiological study on the factors including genetic polymorphism influencing ALT >30 U/L and liver fibrosis progression in metabolic dysfunction-associated steatotic liver disease among the general population.JGH Open. 2024 Dec 19;8(12):e70043. doi: 10.1002/jgh3.70043. eCollection 2024 Dec. JGH Open. 2024. PMID: 39713746 Free PMC article.
-
Genetic influences on circulating retinol and its relationship to human health.Nat Commun. 2024 Feb 19;15(1):1490. doi: 10.1038/s41467-024-45779-x. Nat Commun. 2024. PMID: 38374065 Free PMC article.
-
Increasing hepatic glycogen moderates the diabetic phenotype in insulin-deficient Akita mice.J Biol Chem. 2021 Jan-Jun;296:100498. doi: 10.1016/j.jbc.2021.100498. Epub 2021 Mar 2. J Biol Chem. 2021. PMID: 33667544 Free PMC article.
References
-
- Roach P. J. (2002) Glycogen and its metabolism. Curr. Mol. Med. 2, 101–120 - PubMed
-
- Stalmans W., Bollen M., and Mvumbi L. (1987) Control of glycogen synthesis in health and disease. Diabetes Metab. Rev. 3, 127–161 - PubMed
-
- Villar-Palasí C., and Guinovart J. J. (1997) The role of glucose 6-phosphate in the control of glycogen synthase. FASEB J. 11, 544–558 - PubMed
-
- Newgard C. B., Brady M. J., O'Doherty R. M., and Saltiel A. R. (2000) Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes 49, 1967–1977 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

