Selective Vulnerability of Striatal D2 versus D1 Dopamine Receptor-Expressing Medium Spiny Neurons in HIV-1 Tat Transgenic Male Mice
- PMID: 28473642
- PMCID: PMC5469310
- DOI: 10.1523/JNEUROSCI.0622-17.2017
Selective Vulnerability of Striatal D2 versus D1 Dopamine Receptor-Expressing Medium Spiny Neurons in HIV-1 Tat Transgenic Male Mice
Abstract
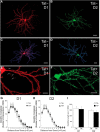
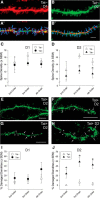
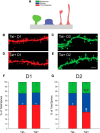
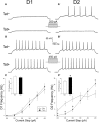
Despite marked regional differences in HIV susceptibility within the CNS, there has been surprisingly little exploration into the differential vulnerability among neuron types and the circuits they underlie. The dorsal striatum is especially susceptible, harboring high viral loads and displaying marked neuropathology, with motor impairment a frequent manifestation of chronic infection. However, little is known about the response of individual striatal neuron types to HIV or how this disrupts function. Therefore, we investigated the morphological and electrophysiological effects of HIV-1 trans-activator of transcription (Tat) in dopamine subtype 1 (D1) and dopamine subtype 2 (D2) receptor-expressing striatal medium spiny neurons (MSNs) by breeding transgenic Tat-expressing mice to Drd1a-tdTomato- or Drd2-eGFP-reporter mice. An additional goal was to examine neuronal vulnerability early during the degenerative process to gain insight into key events underlying the neuropathogenesis. In D2 MSNs, exposure to HIV-1 Tat reduced dendritic spine density significantly, increased dendritic damage (characterized by swellings/varicosities), and dysregulated neuronal excitability (decreased firing at 200-300 pA and increased firing rates at 450 pA), whereas insignificant morphologic and electrophysiological consequences were observed in Tat-exposed D1 MSNs. These changes were concomitant with an increased anxiety-like behavioral profile (lower latencies to enter a dark chamber in a light-dark transition task, a greater frequency of light-dark transitions, and reduced rearing time in an open field), whereas locomotor behavior was unaffected by 2 weeks of Tat induction. Our findings suggest that D2 MSNs and a specific subset of neural circuits within the dorsal striatum are preferentially vulnerable to HIV-1.SIGNIFICANCE STATEMENT Despite combination antiretroviral therapy (cART), neurocognitive disorders afflict 30-50% of HIV-infected individuals and synaptodendritic injury remains evident in specific brain regions such as the dorsal striatum. A possible explanation for the sustained neuronal injury is that the neurotoxic HIV-1 regulatory protein trans-activator of transcription (Tat) continues to be expressed in virally suppressed patients on cART. Using inducible Tat-expressing transgenic mice, we found that dopamine subtype 2 (D2) receptor-expressing medium spiny neurons (MSNs) are selectively vulnerable to Tat exposure compared with D1 receptor-expressing MSNs. This includes Tat-induced reductions in D2 MSN dendritic spine density, increased dendritic damage, and disruptions in neuronal excitability, which coincide with elevated anxiety-like behavior. These data suggest that D2 MSNs and specific circuits within the basal ganglia are preferentially vulnerable to HIV-1.
Keywords: Drd1a-tdTomato-expressing neurons; Drd2-eGFP-expressing neurons; basal ganglia; striatal indirect pathway; synaptic dysfunction; whole-cell patch-clamp physiology.
Copyright © 2017 the authors 0270-6474/17/375759-12$15.00/0.
Figures
Similar articles
-
Progressive Degeneration and Adaptive Excitability in Dopamine D1 and D2 Receptor-Expressing Striatal Neurons Exposed to HIV-1 Tat and Morphine.Cell Mol Neurobiol. 2023 Apr;43(3):1105-1127. doi: 10.1007/s10571-022-01232-5. Epub 2022 Jun 13. Cell Mol Neurobiol. 2023. PMID: 35695980 Free PMC article.
-
Restoration of KCC2 Membrane Localization in Striatal Dopamine D2 Receptor-Expressing Medium Spiny Neurons Rescues Locomotor Deficits in HIV Tat-Transgenic Mice.ASN Neuro. 2021 Jan-Dec;13:17590914211022089. doi: 10.1177/17590914211022089. ASN Neuro. 2021. PMID: 34445881 Free PMC article.
-
Striatal Distribution and Cytoarchitecture of Dopamine Receptor Subtype 1 and 2: Evidence from Double-Labeling Transgenic Mice.Front Neural Circuits. 2017 Aug 17;11:57. doi: 10.3389/fncir.2017.00057. eCollection 2017. Front Neural Circuits. 2017. PMID: 28860974 Free PMC article.
-
D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review.
-
Reappraising striatal D1- and D2-neurons in reward and aversion.Neurosci Biobehav Rev. 2016 Sep;68:370-386. doi: 10.1016/j.neubiorev.2016.05.021. Epub 2016 May 24. Neurosci Biobehav Rev. 2016. PMID: 27235078 Review.
Cited by
-
S-Equol mitigates motivational deficits and dysregulation associated with HIV-1.Sci Rep. 2021 Jun 4;11(1):11870. doi: 10.1038/s41598-021-91240-0. Sci Rep. 2021. PMID: 34088932 Free PMC article.
-
Advances in the Experimental Models of HIV-Associated Neurological Disorders.Curr HIV/AIDS Rep. 2021 Oct;18(5):459-474. doi: 10.1007/s11904-021-00570-1. Epub 2021 Aug 24. Curr HIV/AIDS Rep. 2021. PMID: 34427869 Free PMC article. Review.
-
Astrocytes: Role in pathogenesis and effect of commonly misused drugs in the HIV infected brain.Curr Res Neurobiol. 2023 Aug 29;5:100108. doi: 10.1016/j.crneur.2023.100108. eCollection 2023. Curr Res Neurobiol. 2023. PMID: 38020814 Free PMC article. Review.
-
Mutations of tyrosine 467 in the human norepinephrine transporter attenuate HIV-1 Tat-induced inhibition of dopamine transport while retaining physiological function.PLoS One. 2022 Sep 28;17(9):e0275182. doi: 10.1371/journal.pone.0275182. eCollection 2022. PLoS One. 2022. PMID: 36170295 Free PMC article.
-
Synaptic dysfunction is associated with alterations in the initiation of goal-directed behaviors: Implications for HIV-1-associated apathy.Exp Neurol. 2022 Nov;357:114174. doi: 10.1016/j.expneurol.2022.114174. Epub 2022 Jul 18. Exp Neurol. 2022. PMID: 35863502 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
