A short artificial antimicrobial peptide shows potential to prevent or treat bone infections
- PMID: 28473710
- PMCID: PMC5431435
- DOI: 10.1038/s41598-017-01698-0
A short artificial antimicrobial peptide shows potential to prevent or treat bone infections
Abstract
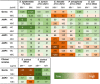
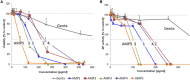
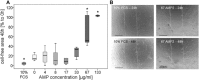
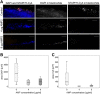
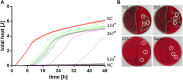
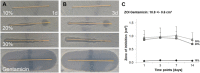
Infection of bone is a severe complication due to the variety of bacteria causing it, their resistance against classical antibiotics, the formation of a biofilm and the difficulty to eradicate it. Antimicrobial peptides (AMPs) are naturally occurring peptides and promising candidates for treatment of joint infections. This study aimed to analyze the effect of short artificial peptides derived from an optimized library regarding (1) antimicrobial effect on different bacterial species, (2) efficacy on biofilms, and (3) effect on osteoblast‑like cells. Culturing the AMP-modifications with Escherichia coli, Enterococcus faecalis, Pseudomonas aeruginosa, Staphylococcus aureus (including clinical isolates of MRSA and MSSA) and Staphylococcus epidermidis identified one candidate that was most effective against all bacteria. This AMP was also able to reduce biofilm as demonstrated by FISH and microcalorimetry. Osteoblast viability and differentiation were not negatively affected by the AMP. A cation concentration comparable to that physiologically occurring in blood had almost no negative effect on AMP activity and even with 10% serum bacterial growth was inhibited. Bacteria internalized into osteoblasts were reduced by the AMP. Taken together the results demonstrate a high antimicrobial activity of the AMP even against bacteria incorporated in a biofilm or internalized into cells without harming human osteoblasts.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
High in vitro antimicrobial activity of synthetic antimicrobial peptidomimetics against staphylococcal biofilms.J Antimicrob Chemother. 2009 Jan;63(1):136-45. doi: 10.1093/jac/dkn464. Epub 2008 Nov 14. J Antimicrob Chemother. 2009. PMID: 19010828
-
In vitro anti-biofilm activity of a biphasic gentamicin-loaded calcium sulfate/hydroxyapatite bone graft substitute.Colloids Surf B Biointerfaces. 2018 Jan 1;161:252-260. doi: 10.1016/j.colsurfb.2017.10.050. Epub 2017 Oct 18. Colloids Surf B Biointerfaces. 2018. PMID: 29096369
-
Design and membrane-disruption mechanism of charge-enriched AMPs exhibiting cell selectivity, high-salt resistance, and anti-biofilm properties.Amino Acids. 2016 Feb;48(2):505-22. doi: 10.1007/s00726-015-2104-0. Epub 2015 Oct 8. Amino Acids. 2016. PMID: 26450121
-
Antimicrobial Peptides as Anti-Infectives against Staphylococcus epidermidis.Med Princ Pract. 2016;25(4):301-8. doi: 10.1159/000443479. Epub 2015 Dec 18. Med Princ Pract. 2016. PMID: 26684017 Free PMC article. Review.
-
Anti-biofilm peptides as a new weapon in antimicrobial warfare.Curr Opin Microbiol. 2016 Oct;33:35-40. doi: 10.1016/j.mib.2016.05.016. Epub 2016 Jun 16. Curr Opin Microbiol. 2016. PMID: 27318321 Free PMC article. Review.
Cited by
-
Perspectives in Searching Antimicrobial Peptides (AMPs) Produced by the Microbiota.Microb Ecol. 2023 Dec 1;87(1):8. doi: 10.1007/s00248-023-02313-8. Microb Ecol. 2023. PMID: 38036921 Free PMC article. Review.
-
The evolution of clinical guidelines for antimicrobial photodynamic therapy of skin.Photochem Photobiol Sci. 2022 Mar;21(3):385-395. doi: 10.1007/s43630-021-00169-w. Epub 2022 Feb 7. Photochem Photobiol Sci. 2022. PMID: 35132604 Free PMC article. Review.
-
Recent advances in musculoskeletal local drug delivery.Acta Biomater. 2019 Jul 15;93:135-151. doi: 10.1016/j.actbio.2019.01.043. Epub 2019 Jan 24. Acta Biomater. 2019. PMID: 30685475 Free PMC article. Review.
-
Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management.Pathogens. 2024 Jan 15;13(1):76. doi: 10.3390/pathogens13010076. Pathogens. 2024. PMID: 38251383 Free PMC article. Review.
-
Uncovering a Hub Signaling Pathway of Antimicrobial-Antifungal-Anticancer Peptides' Axis on Short Cationic Peptides via Network Pharmacology Study.Int J Mol Sci. 2022 Feb 12;23(4):2055. doi: 10.3390/ijms23042055. Int J Mol Sci. 2022. PMID: 35216171 Free PMC article.
References
-
- Arciola CR, et al. Interactions of staphylococci with osteoblasts and phagocytes in the pathogenesis of implant-associated osteomyelitis. Int. J. Artif. Organs. 2012;35(10):713–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

