The Complement System: A Prey of Trypanosoma cruzi
- PMID: 28473804
- PMCID: PMC5397499
- DOI: 10.3389/fmicb.2017.00607
The Complement System: A Prey of Trypanosoma cruzi
Abstract
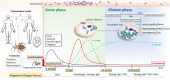
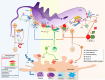
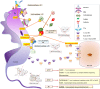
Trypanosoma cruzi is a protozoan parasite known to cause Chagas disease (CD), a neglected sickness that affects around 6-8 million people worldwide. Originally, CD was mainly found in Latin America but more recently, it has been spread to countries in North America, Asia, and Europe due the international migration from endemic areas. Thus, at present CD represents an important concern of global public health. Most of individuals that are infected by T. cruzi may remain in asymptomatic form all lifelong, but up to 40% of them will develop cardiomyopathy, digestive mega syndromes, or both. The interaction between the T. cruzi infective forms and host-related immune factors represents a key point for a better understanding of the physiopathology of CD. In this context, the complement, as one of the first line of host defense against infection was shown to play an important role in recognizing T. cruzi metacyclic trypomastigotes and in controlling parasite invasion. The complement consists of at least 35 or more plasma proteins and cell surface receptors/regulators, which can be activated by three pathways: classical (CP), lectin (LP), and alternative (AP). The CP and LP are mainly initiated by immune complexes or pathogen-associated molecular patterns (PAMPs), respectively, whereas AP is spontaneously activated by hydrolysis of C3. Once activated, several relevant complement functions are generated which include opsonization and phagocytosis of particles or microorganisms and cell lysis. An important step during T. cruzi infection is when intracellular trypomastigotes are release to bloodstream where they may be target by complement. Nevertheless, the parasite uses a sequence of events in order to escape from complement-mediated lysis. In fact, several T. cruzi molecules are known to interfere in the initiation of all three pathways and in the assembly of C3 convertase, a key step in the activation of complement. Moreover, T. cruzi promotes secretion of plasma membrane-derived vesicles from host cells, which prevent the activity of C3 convertase C4b2a and thereby may hinder complement. In this review, we aim to present an overview on the strategies used by T. cruzi in order to circumvent the activation of complement and, consequently, its biological effects.
Keywords: Trypanosoma cruzi; complement regulatory proteins; complement system; evasion mechanism; innate immunity.
Figures

Similar articles
-
Trypanosoma cruzi Evades the Complement System as an Efficient Strategy to Survive in the Mammalian Host: The Specific Roles of Host/Parasite Molecules and Trypanosoma cruzi Calreticulin.Front Microbiol. 2017 Sep 1;8:1667. doi: 10.3389/fmicb.2017.01667. eCollection 2017. Front Microbiol. 2017. PMID: 28919885 Free PMC article. Review.
-
Reevaluating the Trypanosoma cruzi proteomic map: The shotgun description of bloodstream trypomastigotes.J Proteomics. 2015 Feb 6;115:58-65. doi: 10.1016/j.jprot.2014.12.003. Epub 2014 Dec 20. J Proteomics. 2015. PMID: 25534883
-
Is It Possible to Intervene in the Capacity of Trypanosoma cruzi to Elicit and Evade the Complement System?Front Immunol. 2021 Dec 16;12:789145. doi: 10.3389/fimmu.2021.789145. eCollection 2021. Front Immunol. 2021. PMID: 34975884 Free PMC article. Review.
-
Inefficient complement system clearance of Trypanosoma cruzi metacyclic trypomastigotes enables resistant strains to invade eukaryotic cells.PLoS One. 2010 Mar 16;5(3):e9721. doi: 10.1371/journal.pone.0009721. PLoS One. 2010. PMID: 20300530 Free PMC article.
-
Evasion of the Immune Response by Trypanosoma cruzi during Acute Infection.Front Immunol. 2016 Jan 18;6:659. doi: 10.3389/fimmu.2015.00659. eCollection 2015. Front Immunol. 2016. PMID: 26834737 Free PMC article. Review.
Cited by
-
Toxoplasma gondii Mechanisms of Entry Into Host Cells.Front Cell Infect Microbiol. 2020 Jun 30;10:294. doi: 10.3389/fcimb.2020.00294. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32714877 Free PMC article.
-
Mechanisms by which Factor H protects Trypanosoma cruzi from the alternative pathway of complement.Front Immunol. 2024 Feb 1;15:1152000. doi: 10.3389/fimmu.2024.1152000. eCollection 2024. Front Immunol. 2024. PMID: 38361922 Free PMC article.
-
Pathology and Pathogenesis of Chagas Heart Disease.Annu Rev Pathol. 2019 Jan 24;14:421-447. doi: 10.1146/annurev-pathol-020117-043711. Epub 2018 Oct 24. Annu Rev Pathol. 2019. PMID: 30355152 Free PMC article. Review.
-
Animal models for exploring Chagas disease pathogenesis and supporting drug discovery.Clin Microbiol Rev. 2024 Dec 10;37(4):e0015523. doi: 10.1128/cmr.00155-23. Epub 2024 Nov 15. Clin Microbiol Rev. 2024. PMID: 39545730 Review.
-
Comparative Analysis of Virulence Mechanisms of Trypanosomatids Pathogenic to Humans.Front Cell Infect Microbiol. 2021 Apr 16;11:669079. doi: 10.3389/fcimb.2021.669079. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33937106 Free PMC article. Review.
References
-
- Aguillón J. C., Ferreira L., Pérez C., Colombo A., Molina M. C., Wallace A., et al. (2000). Tc45, a dimorphic Trypanosoma cruzi immunogen with variable chromosomal localization, is calreticulin. Am. J. Trop. Med. Hyg. 63 306–312. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

