The radial organization of neuronal primary cilia is acutely disrupted by seizure and ischemic brain injury
- PMID: 28473847
- PMCID: PMC5412953
- DOI: 10.1007/s11515-017-1447-1
The radial organization of neuronal primary cilia is acutely disrupted by seizure and ischemic brain injury
Abstract
Background: Neuronal primary cilia are sensory organelles that are critically involved in the proper growth, development, and function of the central nervous system (CNS). Recent work also suggests that they signal in the context of CNS injury, and that abnormal ciliary signaling may be implicated in neurological diseases.
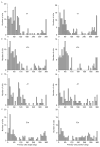
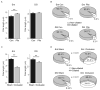
Methods: We quantified the distribution of neuronal primary cilia alignment throughout the normal adult mouse brain by immunohistochemical staining for the primary cilia marker adenylyl cyclase III (ACIII) and measuring the angles of primary cilia with respect to global and local coordinate planes. We then introduced two different models of acute brain insult-temporal lobe seizure and cerebral ischemia, and re-examined neuronal primary cilia distribution, as well as ciliary lengths and the proportion of neurons harboring cilia.
Results: Under basal conditions, cortical cilia align themselves radially with respect to the cortical surface, while cilia in the dentate gyrus align themselves radially with respect to the granule cell layer. Cilia of neurons in the striatum and thalamus, by contrast, exhibit a wide distribution of ciliary arrangements. In both cases of acute brain insult, primary cilia alignment was significantly disrupted in a region-specific manner, with areas affected by the insult preferentially disrupted. Further, the two models promoted differential effects on ciliary lengths, while only the ischemia model decreased the proportion of ciliated cells.
Conclusions: These findings provide evidence for the regional anatomical organization of neuronal primary cilia in the adult brain and suggest that various brain insults may disrupt this organization.
Keywords: cerebral cortex; cerebral ischemia; dentate gyrus; temporal lobe seizure.
Figures







Similar articles
-
Neonatal seizures induced by pentylenetetrazol or kainic acid disrupt primary cilia growth on developing mouse cortical neurons.Exp Neurol. 2016 Aug;282:119-27. doi: 10.1016/j.expneurol.2016.05.015. Epub 2016 May 13. Exp Neurol. 2016. PMID: 27181411
-
Type 3 Adenylyl Cyclase and Somatostatin Receptor 3 Expression Persists in Aged Rat Neocortical and Hippocampal Neuronal Cilia.Front Aging Neurosci. 2016 May 31;8:127. doi: 10.3389/fnagi.2016.00127. eCollection 2016. Front Aging Neurosci. 2016. PMID: 27303293 Free PMC article.
-
Quantitative Comparison of Primary Cilia Marker Expression and Length in the Mouse Brain.J Mol Neurosci. 2018 Mar;64(3):397-409. doi: 10.1007/s12031-018-1036-z. Epub 2018 Feb 20. J Mol Neurosci. 2018. PMID: 29464516
-
Influences of primary cilia on cortical morphogenesis and neuronal subtype maturation.Neuroscientist. 2015 Apr;21(2):136-51. doi: 10.1177/1073858414531074. Epub 2014 Apr 16. Neuroscientist. 2015. PMID: 24740576 Review.
-
Type 3 adenylyl cyclase: a key enzyme mediating the cAMP signaling in neuronal cilia.Int J Physiol Pathophysiol Pharmacol. 2016 Sep 30;8(3):95-108. eCollection 2016. Int J Physiol Pathophysiol Pharmacol. 2016. PMID: 27785336 Free PMC article. Review.
Cited by
-
Nanometer-scale views of visual cortex reveal anatomical features of primary cilia poised to detect synaptic spillover.bioRxiv [Preprint]. 2023 Nov 6:2023.10.31.564838. doi: 10.1101/2023.10.31.564838. bioRxiv. 2023. Update in: Curr Biol. 2024 Jun 3;34(11):2418-2433.e4. doi: 10.1016/j.cub.2024.04.043. PMID: 37961618 Free PMC article. Updated. Preprint.
-
Seizure enhances SUMOylation and zinc-finger transcriptional repression in neuronal nuclei.iScience. 2023 Aug 23;26(9):107707. doi: 10.1016/j.isci.2023.107707. eCollection 2023 Sep 15. iScience. 2023. PMID: 37694138 Free PMC article.
-
Fbxo41 Promotes Disassembly of Neuronal Primary Cilia.Sci Rep. 2019 Jun 3;9(1):8179. doi: 10.1038/s41598-019-44589-2. Sci Rep. 2019. PMID: 31160656 Free PMC article.
-
A serotonergic axon-cilium synapse drives nuclear signaling to alter chromatin accessibility.Cell. 2022 Sep 1;185(18):3390-3407.e18. doi: 10.1016/j.cell.2022.07.026. Cell. 2022. PMID: 36055200 Free PMC article.
-
[Pulsed electromagnetic fields stimulating osteogenic differentiation and maturation involves primary cilia-PI3K/AKT pathway].Beijing Da Xue Xue Bao Yi Xue Ban. 2019 Apr 18;51(2):245-251. doi: 10.19723/j.issn.1671-167X.2019.02.008. Beijing Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 30996361 Free PMC article. Chinese.
References
-
- Albrecht PJ, Dahl JP, Stoltzfus OK, Levenson R, Levison SW. Ciliary neurotrophic factor activates spinal cord astrocytes, stimulating their production and release of fibroblast growth factor-2, to increase motor neuron survival. Exp Neurol. 2002;173(1):46–62. - PubMed
-
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25(1):1–27. - PubMed
-
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol. 2007;505(5):562–571. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
