Economic impact of biologic utilization patterns in patients with psoriatic arthritis
- PMID: 28474139
- PMCID: PMC5486473
- DOI: 10.1007/s10067-017-3636-3
Economic impact of biologic utilization patterns in patients with psoriatic arthritis
Abstract
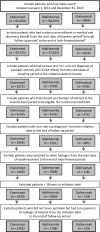
The aim of the study is to examine the frequency and costs associated with above-label dosing of biologics in patients with psoriatic arthritis (PsA). MarketScan identified adults with ≥1 International Classification of Diseases, Clinical Modification diagnosis for PsA and ≥1 pharmacy claim for biologics of interest between January 1, 2011 and December 31, 2013. The first biologic claim was the index date with a 1-year follow-up period and three additional months to confirm continuous biologic use. Exclusion criteria included switching to a different biologic or diagnosis with another autoimmune disease. During the follow-up period, duration was stratified into three groups: <30, 30-179, and ≥180 days of above-label dosing (>10% of the labeled dose). One-tailed t test was conducted to examine the impact of above-label duration on healthcare costs. We identified 4245 PsA patients receiving etanercept (n = 2342), adalimumab (n = 1788), and golimumab (n = 115). Above-label dosing of <30 days (85% adalimumab, 90.4% etanercept, and 95.7% golimumab) and ≥180 days (9.6% adalimumab, 4.1% etanercept, and 2.6% golimumab) was observed. All-cause total healthcare costs for <30 days of above-label use (etanercept $30,625, adalimumab $31,620, and golimumab $37,224), 30-179 days (etanercept $35,602, adalimumab $38,915, and golimumab $64,349), and ≥180 days (etanercept $55,349, adalimumab $54,176, and golimumab $47,993) were reported. Longer above-label duration (30-179 versus <30 days, ≥180 versus 30-179 and ≥180 days) with etanercept or adalimumab was significantly associated with higher mean increased total all-cause healthcare, PsA-specific healthcare, and biologic costs (p < 0.05). Above-label use of anti-TNF biologics does occur and is associated with significantly increased healthcare costs.
Keywords: Above-label dosing; Disease-modifying antirheumatic drug; Dose escalation; Drug costs; Psoriatic arthritis.
Conflict of interest statement
Funding for this study was provided by Novartis Pharmaceuticals Corporation. S. Schwartzman works at the Hospital for Special Surgery, New York, NY, and is a consultant for Abbvie, Crescendo, Epirus, Genentech, Janssen, Novartis, Hospira, Pfizer, UCB, Discus Analytics, and the National Psoriasis Foundation. Y. Li and J.B. Palmer are employees of Novartis Pharmaceuticals Corporation. H. Zhou is an Analyst at KMK Consulting Inc. and works as a consultant for Novartis Pharmaceuticals Corporation.
Figures
References
-
- American Academy of Dermatology Working Group. Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65:137–174. doi: 10.1016/j.jaad.2010.11.055. - DOI - PubMed
-
- Day MS, Nam D, Goodman S, Su EP, Figgie M. Psoriatic arthritis. J Am Acad Orthop Surg. 2012;20:28–37. - PubMed
-
- Ogdie-Beatty AR. GRAPPA initiatives continue to chart the way for psoriasis, psoriatic arthritis. PM360-Rheumatology. Available from: http://www.pm360online.com/grappa-initiatives-continue-to-chart-the-way-.... Internet. Accessed 30 June 2015
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

