Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells
- PMID: 28474622
- PMCID: PMC5433278
- DOI: 10.4103/ijmr.IJMR_1842_15
Preclinical safety & toxicity evaluation of pooled, allogeneic human bone marrow-derived mesenchymal stromal cells
Abstract
Background & objectives: Administration of ex vivo-expanded human bone marrow-derived mesenchymal stromal cells (hBMMSC) obtained from single donors has shown therapeutic benefits in both preclinical and clinical studies. In this study, the safety, toxicity and biodistribution profiles of a pooled hBMMSC population, produced from three healthy donors were assessed in rodent and non-rodents.
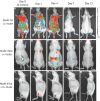
Methods: The pooled hBMMSC population was characterized by their expression of various cell surface markers, differentiation potential and immunomodulatory activity. To establish in vivo safety of the pooled cells, these were administered by various injection routes into rodents and non-rodents to determine overall toxicity, biodistribution and tumorigenic potential in a series of preclinical studies.
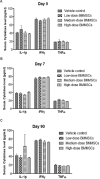
Results: Single injections of hBMMSC at various doses through intravenous or intramuscular routes did not cause toxicity in rats and rabbits. In addition, repeat administration of hBMMSC was also well tolerated by rats, and no prenatal toxicity was observed by multiple administration in the same animal species. Ex vivo-expanded and cryopreserved hBMMSCs did not induce tumour formation in severe combined immunodeficient (SCID) mice.
Interpretation & conclusions: Our results showed that the pooled hBMMSC population was non-toxic, non-teratogenic and non-tumorigenic in animals. Further studies need to be done to find out if it can be safely administered in human patients.
Conflict of interest statement
Figures


References
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. - PubMed
-
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
