Nickel-catalysed retro-hydroamidocarbonylation of aliphatic amides to olefins
- PMID: 28474671
- PMCID: PMC5424121
- DOI: 10.1038/ncomms14993
Nickel-catalysed retro-hydroamidocarbonylation of aliphatic amides to olefins
Abstract
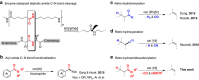
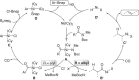
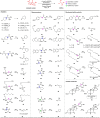
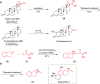
Amide and olefins are important synthetic intermediates with complementary reactivity which play a key role in the construction of natural products, pharmaceuticals and manmade materials. Converting the normally highly stable aliphatic amides into olefins directly is a challenging task. Here we show that a Ni/NHC-catalytic system has been established for decarbonylative elimination of aliphatic amides to generate various olefins via C-N and C-C bond cleavage. This study not only overcomes the acyl C-N bond activation in aliphatic amides, but also encompasses distinct chemical advances on a new type of elimination reaction called retro-hydroamidocarbonylation. This transformation shows good functional group compatibility and can serve as a powerful synthetic tool for late-stage olefination of amide groups in complex compounds.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Nickel-Catalyzed Decarbonylative Borylation of Amides: Evidence for Acyl C-N Bond Activation.Angew Chem Int Ed Engl. 2016 Jul 18;55(30):8718-22. doi: 10.1002/anie.201603068. Epub 2016 Jun 3. Angew Chem Int Ed Engl. 2016. PMID: 27258597
-
Factors Controlling the Reactivity and Chemoselectivity of Resonance Destabilized Amides in Ni-Catalyzed Decarbonylative and Nondecarbonylative Suzuki-Miyaura Coupling.J Am Chem Soc. 2017 Nov 1;139(43):15522-15529. doi: 10.1021/jacs.7b09482. Epub 2017 Oct 20. J Am Chem Soc. 2017. PMID: 29017320
-
Nickel-Catalyzed Esterification of Aliphatic Amides.Angew Chem Int Ed Engl. 2016 Nov 21;55(48):15129-15132. doi: 10.1002/anie.201607856. Epub 2016 Nov 4. Angew Chem Int Ed Engl. 2016. PMID: 27813308 Free PMC article.
-
Classes of Amides that Undergo Selective N-C Amide Bond Activation: The Emergence of Ground-State Destabilization.J Org Chem. 2023 Oct 6;88(19):13371-13391. doi: 10.1021/acs.joc.2c01094. Epub 2022 Sep 2. J Org Chem. 2023. PMID: 36054817 Review.
-
Well-Defined Pre-Catalysts in Amide and Ester Bond Activation.Molecules. 2019 Jan 9;24(2):215. doi: 10.3390/molecules24020215. Molecules. 2019. PMID: 30634382 Free PMC article. Review.
Cited by
-
Olefination via Cu-Mediated Dehydroacylation of Unstrained Ketones.J Am Chem Soc. 2021 Dec 8;143(48):20042-20048. doi: 10.1021/jacs.1c09587. Epub 2021 Nov 22. J Am Chem Soc. 2021. PMID: 34807585 Free PMC article.
-
Activation of C-O and C-N Bonds Using Non-Precious-Metal Catalysis.ACS Catal. 2020 Oct 16;10(20):12109-12126. doi: 10.1021/acscatal.0c03334. Epub 2020 Sep 10. ACS Catal. 2020. PMID: 33868770 Free PMC article. No abstract available.
-
A donor-acceptor complex enables the synthesis of E-olefins from alcohols, amines and carboxylic acids.Chem Sci. 2021 Apr 5;12(19):6684-6690. doi: 10.1039/d1sc01024g. Chem Sci. 2021. PMID: 34040742 Free PMC article.
-
Nickel-Catalyzed Suzuki-Miyaura Coupling of Aliphatic Amides.ACS Catal. 2018 Feb 2;8(2):1003-1008. doi: 10.1021/acscatal.7b03688. Epub 2017 Dec 20. ACS Catal. 2018. PMID: 29682398 Free PMC article.
-
Mechanistic insights on C(acyl)-N functionalisation mediated by late transition metals.Dalton Trans. 2024 Dec 3;53(47):18803-18818. doi: 10.1039/d4dt01829j. Dalton Trans. 2024. PMID: 39115156 Review.
References
-
- Negoro S. Biodegradation of nylon oligomers. Appl. Microbiol. Biotechnol. 54, 461–466 (2000). - PubMed
-
- Nahm S. & Weinreb S. M. N-Methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 22, 3815–3818 (1981).
-
- Weires N. A., Baker E. L. & Garg N. K. Nickel-catalysed Suzuki–Miyaura coupling of amides. Nat. Chem. 8, 75–79 (2016). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

