Connexin36 localization to pinealocytes in the pineal gland of mouse and rat
- PMID: 28474748
- PMCID: PMC5507615
- DOI: 10.1111/ejn.13602
Connexin36 localization to pinealocytes in the pineal gland of mouse and rat
Abstract
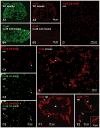
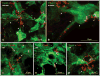
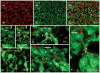
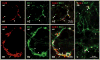
Several cell types in the pineal gland are known to establish intercellular gap junctions, but the connexin constituents of those junctions have not been fully characterized. Specifically, the expression of connexin36 (Cx36) protein and mRNA has been examined in the pineal, but the identity of cells that produce Cx36 and that form Cx36-containing gap junctions has not been determined. We used immunofluorescence and freeze fracture replica immunogold labelling (FRIL) of Cx36 to investigate the cellular and subcellular localization of Cx36 in the pineal gland of adult mouse and rat. Immunofluorescence labelling of Cx36 was visualized exclusively as puncta or short immunopositive strands that were distributed throughout the pineal, and which were absent in pineal sections from Cx36 null mice. By double immunofluorescence labelling, Cx36 was localized to tryptophan hydroxylase-positive and 5-hydroxytryptamine-positive pinealocyte cell bodies and their large initial processes, including at intersections of those processes and at sites displaying a confluence of processes. Labelling for the cell junction marker zonula occludens-1 (ZO-1) either overlapped or was closely associated with labelling for Cx36. Pinealocytes thus form Cx36-containing gap junctions that also incorporate the scaffolding protein ZO-1. FRIL revealed labelling of Cx36 at ultrastructurally defined gap junctions between pinealocytes, most of which was at gap junctions having reticular, ribbon or string configurations. The results suggest that the endocrine functions of pinealocytes and their secretion of melatonin is supported by their intercellular communication via Cx36-containing gap junctions, which may now be tested by the use of Cx36 null mice.
Keywords: gap junctions; immunofluorescence; melatonin; zonula occludens-1.
© 2017 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- Belluardo N, Mudo G, Trovato-Salinaro A, le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. - PubMed
-
- Berthoud VM, Saez JC. Changes in connexin43, the gap junction protein of astrocytes, during development of the rat pineal gland. J Pineal Res. 1993;14:67–72. - PubMed
-
- Berthoud VM, Hall DH, Strahsburger E, Beyer EC, Saez JC. Gap junctions in the chicken pineal gland. Brain Res. 2000;861:257–270. - PubMed
-
- Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev. 2011;91:1393–1445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

