In vivo targeting of protein antigens to dendritic cells using anti-DEC-205 single chain antibody improves HIV Gag specific CD4+ T cell responses protecting from airway challenge with recombinant vaccinia-gag virus
- PMID: 28474788
- PMCID: PMC6485703
- DOI: 10.1002/iid3.151
In vivo targeting of protein antigens to dendritic cells using anti-DEC-205 single chain antibody improves HIV Gag specific CD4+ T cell responses protecting from airway challenge with recombinant vaccinia-gag virus
Abstract
Introduction: Targeting antigens to dendritic cells (DCs) in vivo via a DC-restricted endocytic receptor, DEC205, has been validated to enhance immunity in several vaccine platforms. Particularly atttractive is selected delivery of proteins to DCs in vivo because it enables proteins to be more immunogenic and provides a cheaper and effective way for repeated immunizations.
Methods: In this study, we tested the efficacy of a single chain antibody to DEC205 (scDEC) to deliver protein antigens selectively to DCs in vivo and to induce protective immunity.
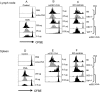
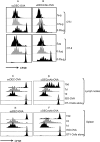
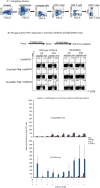
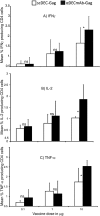
Results: In comparison to soluble Ovalbumin (OVA) antigen, when recombinant scDEC:OVA protein was injected subcutaneously (s.c.) into mice, the OVA protein was selectively presented by DCs to both TCR transgenic CD8+ and CD4+ T cells approximately 500 and 100 times more efficient than soluble OVA, respectively, and could persist for seven days following s.c. injection of the scDEC205:OVA. Similarly selective targeting of HIV Gag P24 to DCs in vivo using scDEC-Gag protein plus polyICLC vaccine resulted in strong, long lasting, polyfuntional CD4+ T cells in mice which were protective against airway challenge by a recombinant vaccinia-gag virus.
Conclusion: Thus targeting protein antigens to DCs using scDEC can be used either alone or in combination with other strategies for effective immunization.
Keywords: Antigens; dendritic cells; immunization.
© 2017 The Authors. Immunity, Inflammation and Disease Published by John Wiley & Sons Ltd.
Figures


Similar articles
-
Dendritic cell targeted HIV-1 gag protein vaccine provides help to a recombinant Newcastle disease virus vectored vaccine including mobilization of protective CD8+ T cells.Immun Inflamm Dis. 2018 Mar;6(1):163-175. doi: 10.1002/iid3.209. Epub 2017 Dec 4. Immun Inflamm Dis. 2018. PMID: 29205929 Free PMC article.
-
Comparable T helper 1 (Th1) and CD8 T-cell immunity by targeting HIV gag p24 to CD8 dendritic cells within antibodies to Langerin, DEC205, and Clec9A.Proc Natl Acad Sci U S A. 2011 Feb 8;108(6):2384-9. doi: 10.1073/pnas.1019547108. Epub 2011 Jan 24. Proc Natl Acad Sci U S A. 2011. PMID: 21262813 Free PMC article.
-
Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine.J Exp Med. 2006 Mar 20;203(3):607-17. doi: 10.1084/jem.20052005. Epub 2006 Feb 27. J Exp Med. 2006. PMID: 16505141 Free PMC article.
-
Dendritic cell-targeted protein vaccines: a novel approach to induce T-cell immunity.J Intern Med. 2012 Feb;271(2):183-92. doi: 10.1111/j.1365-2796.2011.02496.x. Epub 2012 Jan 4. J Intern Med. 2012. PMID: 22126373 Free PMC article. Review.
-
Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection.Springer Semin Immunopathol. 2006 Nov;28(3):209-19. doi: 10.1007/s00281-006-0025-4. Epub 2006 Aug 25. Springer Semin Immunopathol. 2006. PMID: 16932955 Review. No abstract available.
Cited by
-
Intradermal Delivery of Dendritic Cell-Targeting Chimeric mAbs Genetically Fused to Type 2 Dengue Virus Nonstructural Protein 1.Vaccines (Basel). 2020 Oct 1;8(4):565. doi: 10.3390/vaccines8040565. Vaccines (Basel). 2020. PMID: 33019498 Free PMC article.
-
Induction of Therapeutic Protection in an HPV16-Associated Mouse Tumor Model Through Targeting the Human Papillomavirus-16 E5 Protein to Dendritic Cells.Front Immunol. 2021 Feb 25;12:593161. doi: 10.3389/fimmu.2021.593161. eCollection 2021. Front Immunol. 2021. PMID: 33717073 Free PMC article.
-
Heterologous DNA Prime- Subunit Protein Boost with Chikungunya Virus E2 Induces Neutralizing Antibodies and Cellular-Mediated Immunity.Int J Mol Sci. 2023 Jun 23;24(13):10517. doi: 10.3390/ijms241310517. Int J Mol Sci. 2023. PMID: 37445695 Free PMC article.
-
Heterologous prime-boost vaccination protects against EBV antigen-expressing lymphomas.J Clin Invest. 2019 May 1;129(5):2071-2087. doi: 10.1172/JCI125364. Epub 2019 Apr 15. J Clin Invest. 2019. PMID: 31042161 Free PMC article.
-
Targeted delivery of autoantigen to dendritic cells prevents development of spontaneous uveitis.Front Immunol. 2023 Sep 1;14:1227633. doi: 10.3389/fimmu.2023.1227633. eCollection 2023. Front Immunol. 2023. PMID: 37727784 Free PMC article.
References
-
- Steinman, R. M. 2010. Some active areas of DC research and their medical potential. Eur. J. Immunol. 40:2085–2088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

