The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways
- PMID: 28475177
- PMCID: PMC5520163
- DOI: 10.1038/cdd.2017.17
The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways
Abstract
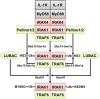
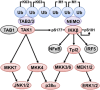
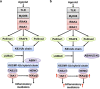
The adaptor protein MyD88 is required for signal transmission by toll-like receptors and receptors of the interleukin-1 family of cytokines. MyD88 signalling triggers the formation of Lys63-linked and Met1-linked ubiquitin (K63-Ub, M1-Ub) chains within minutes. The K63-Ub chains, which are formed by the E3 ubiquitin ligases TRAF6, Pellino1 and Pellino2, activate TAK1, the master kinase that switches on mitogen-activated protein (MAP) kinase cascades and initiates activation of the canonical IκB kinase (IKK) complex. The M1-Ub chains, which are formed by the linear ubiquitin chain assembly complex (LUBAC), bind to the NEMO (NF-κB essential modulator) component of the IKK complex and are required for TAK1 to activate IKKs, but not MAP kinases. An essential E3 ligase-independent role of TRAF6 is to recruit LUBAC into the MyD88 signalling complex, where it recognises preformed K63-Ub chains attached to protein components of these complexes, such as IRAK1 (IL-1 receptor-associated kinase), producing ubiquitin chains containing both types of linkage, termed K63/M1-Ub hybrids. The formation of K63/M1-Ub hybrids, which is a feature of several innate immune signalling pathways, permits the co-recruitment of proteins that interact with either K63-Ub or M1-Ub chains. Two likely roles for K63/M1-Ub hybrids are to facilitate the TAK1-dependent activation of the IKK complex and to prevent the hyperactivation of these kinases by recruiting A20 and A20-binding inhibitor of NF-κB1 (ABIN1). These proteins restrict activation of the TAK1 and IKK complexes, probably by competing with them for binding to K63/M1-Ub hybrids. The formation of K63/M1-Ub hybrids may also regulate the rate at which the ubiquitin linkages in these chains are hydrolysed. The IKK-catalysed phosphorylation of some of its substrates permits their recognition by the E3 ligase SCFβTRCP, leading to their Lys48-linked ubiquitylation and proteasomal degradation. Innate immune signalling is therefore controlled by the formation and destruction of three different types of ubiquitin linkage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK et al. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 1989; 243: 1576–1583. - PubMed
-
- Ciehanover A, Hod Y, Hershko A. A heat-stable polypeptide component of an ATP-dependent proteolytic system from reticulocytes. Biochem Biophys Res Commun 1978; 81: 1100–1105. - PubMed
-
- Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 1999; 96: 645–653. - PubMed
-
- Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature 1996; 383: 443–446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

