Efficacy and Safety of Lurasidone in Adolescents with Schizophrenia: A 6-Week, Randomized Placebo-Controlled Study
- PMID: 28475373
- PMCID: PMC5568017
- DOI: 10.1089/cap.2016.0189
Efficacy and Safety of Lurasidone in Adolescents with Schizophrenia: A 6-Week, Randomized Placebo-Controlled Study
Abstract
Objective: To evaluate the efficacy and safety of lurasidone in acutely symptomatic adolescent patients with schizophrenia.
Methods: Patients aged 13-17 years were randomly assigned to 6 weeks of double-blind, fixed-dose lurasidone (40 or 80 mg/day) or placebo. Primary and key secondary efficacy measures were change from baseline to week 6 in the Positive and Negative Symptom Scale (PANSS) total score and Clinical Global Impressions-Severity (CGI-S) score, respectively, using mixed model for repeated measurement (MMRM) analysis. The proportion of patients achieving treatment response at endpoint, based on ≥20% reduction in PANSS total score, was analyzed using a logistic regression model.
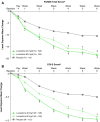
Results: Least-squares (LS) mean change in PANSS total score from baseline to week 6 was -18.6 with lurasidone 40 mg/day (N = 108; p < 0.001 vs. placebo; effect size = 0.51), -18.3 with lurasidone 80 mg/day (N = 106; p < 0.001 vs. placebo; effect size = 0.48), and -10.5 with placebo (N = 112). Similarly, LS mean change in CGI-S score from baseline to week 6 was significantly greater with lurasidone 40 mg/day (-1.0; p < 0.001; effect size = 0.49) and 80 mg/day (-0.9; p = 0.0015; effect size = 0.45) compared with placebo (-0.5). A significantly higher proportion of patients met responder criteria on lurasidone 40 and 80 mg/day versus placebo (63.9% and 65.1% vs. 42.0%; p < 0.001 for both comparisons). The rate of study discontinuation was 10.3% in lurasidone-treated and 17.7% in placebo-treated patients. The most common adverse events (incidence ≥5% in either lurasidone dose group and at least twice the rate of placebo) for lurasidone 40 mg/day, 80 mg/day, and placebo, respectively, were nausea (12.7%, 14.4%, and 2.7%), somnolence (9.1%, 11.5%, and 5.4%), akathisia (9.1%, 8.7%, and 1.8%), vomiting (8.2%, 6.7%, and 1.8%), and sedation (5.5%, 1.9%, and 1.8%). Treatment with lurasidone was not associated with clinically meaningful effects on body weight, lipids, measures of glycemic control, or prolactin.
Conclusions: In this 6-week study, lurasidone at doses of 40 and 80 mg/day demonstrated statistically significant and clinically meaningful symptom improvement in adolescent patients with schizophrenia. Lurasidone was generally well tolerated with few effects on weight and metabolic parameters, consistent with findings in adult patients with schizophrenia.
Keywords: adolescent; clinical study; lurasidone; schizophrenia; treatment.
Conflict of interest statement
Drs. R.G., A.L., J.C., and L.D. are employees of Sunovion Pharmaceuticals, Inc. In the last 36 months, Dr. R.L.F. received or has received research support, acted as a consultant, and/or served on a speaker's bureau for Actavis, Akili, Alcobra, American Academy of Child and Adolescent Psychiatry, American Psychiatric Press, Bracket, CogCubed, Cognition Group, Coronado Biosciences, Elsevier, Epharma Solutions, Forest, Genentech, GlaxoSmithKline, Guilford Press, Ironshore, Johns Hopkins University Press, KemPharm, Lundbeck, Medgenics, Merck, NIH, Neurim, Novartis, Otsuka, PCORI, Pfizer, Physicians Postgraduate Press, Purdue, Rhodes Pharmaceuticals, Roche, Sage, Shire, Sunovion, Supernus Pharmaceuticals, Syneurx, Takeda, Teva, Tris, Validus, and WebMD.
Figures
References
-
- Andreasen NC, Carpenter WT, Jr., Kane JM, et al. : Remission in schizophrenia: Proposed criteria and rationale for consensus. Am J Psychiatry 162:441–449, 2005 - PubMed
-
- Barnes TR: A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676, 1989 - PubMed
-
- Beratis S, Gabriel J, Hoidas S: Age at onset in subtypes of schizophrenic disorders. Schizophr Bull 20:287–296, 1994 - PubMed
-
- Citrome L, Cucchiaro J, Sarma K, Phillips D, Silva R, Tsuchiya S, Loebel A: Long-term safety and tolerability of lurasidone in schizophrenia: A 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 27:165–176, 2012 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
