Blocking Smad2 signalling with loganin attenuates SW10 cell cycle arrest induced by TNF-α
- PMID: 28475649
- PMCID: PMC5419568
- DOI: 10.1371/journal.pone.0176965
Blocking Smad2 signalling with loganin attenuates SW10 cell cycle arrest induced by TNF-α
Abstract
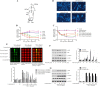
The activity of Schwann cells (SWCs) is very important in trauma-induced nerve repair, and tumour necrosis factor-α (TNF-α) produced during tissue injury inhibits the viability of SWCs, which delays the repair of peripheral nerves. Loganin is an iridoid glycoside that has been shown to alleviate a variety of cytotoxic effects. In the current study, we evaluated the potential efficacy and the mechanism of action of loganin in TNF-α-induced cytotoxicity in SW10 cells. The experimental results indicated that loganin blocked TNF-α-mediated Smad2 activation, downregulated the expression of the G1 phase cell cycle inhibitor p15IN4KB, and upregulated the expression of the G1 phase cell cycle activator cyclin D1-CDK4/6, which upregulated E2F-1-dependent survivin expression and relieved TNF-α-induced apoptosis in SW10 cells. The protective effect of loganin on SWCs has potential medicinal value in the promotion of peripheral nerve repair and is significant for studies in the field of tissue regeneration.
Conflict of interest statement
Figures




References
-
- Kay S. Surgical Disorders of the Peripheral Nerves. Plastic & Reconstructive Surgery. 1973; 105(6):365–366.
-
- Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996; 73(73):625–9. - PubMed
-
- Mulleman D, Mammou S, Griffoul I, Watier H, Goupille P. Pathophysiology of disk-related low back pain and sciatica. II. Evidence supporting treatment with TNF-alpha antagonists. Joint Bone Spine Revue Du Rhumatisme. 2006; 3(3):270–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

