A double-blind, placebo-controlled randomized trial of creatine for the cancer anorexia/weight loss syndrome (N02C4): an Alliance trial
- PMID: 28475678
- PMCID: PMC5808669
- DOI: 10.1093/annonc/mdx232
A double-blind, placebo-controlled randomized trial of creatine for the cancer anorexia/weight loss syndrome (N02C4): an Alliance trial
Abstract
Background: Multiple pilot studies, including one in colorectal cancer patients, suggest that creatine, an amino acid derivative, augments muscle, improves strength, and thereby could palliate the cancer anorexia/weight loss syndrome.
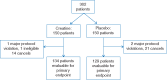
Patients and methods: In this randomized, double-blind, placebo-controlled trial, incurable patients with this syndrome were assigned creatine (20 g/day load×5 days followed by 2 g/day orally) versus identical placebo. Patients were weighed once a week for 1 month and then monthly. Patients were also assessed over 1 month for appetite and quality of life (validated questionnaires), fist grip strength, body composition (bioelectrical impedance), and adverse events. The primary endpoint was 10% or greater weight gain from baseline during the first month.
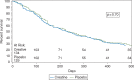
Results: Within this combined cohort of 263 evaluable patients (134 received creatine and 129 placebo), only 3 gained ≥10% of their baseline weight by 1 month: two creatine-treated and the other placebo-exposed (P = 1.00). Questionnaire data on appetite, quality of life, and activities of daily living showed no statistically significant differences between groups. Similarly, no statistically significant differences between groups were observed for fist-grip strength or body composition. Rates and severity of adverse events were comparable between groups. Finally, a median survival of 230 and 239 days were observed in the creatine and placebo groups, respectively (P = 0.70).
Conclusion: Creatine, as prescribed in this trial, had no effect on the cancer anorexia/weight loss syndrome.
Keywords: cachexia; creatine; nutrition; supportive care; weight loss.
© The Author 2017. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Lanhers C, Pereira B, Naughton G. et al. Creatine supplementation and upper limb strength performance: a systemic review and meta-analysis. Sports Med 2017; 47: 163–173. - PubMed
-
- Safdar A, Yardley NJ, Snow R. et al. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Gen 2008; 32: 219–228. - PubMed
-
- Johannsmeyer S, Candow DG, Brahms CM. et al. Effect of creatine supplementation and drop set resistance training in untrained aging adults. Exp Gerontol 2016; 83: 112–119. - PubMed
-
- Wilkinson TJ, Lemmey AB, Jones JG. et al. Can creatine supplementation improve body composition and objective physical function in rheumatoid arthritis patients? A randomized controlled trial. Arthritis Care Res 2016; 68: 729–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

