Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1
- PMID: 28475873
- PMCID: PMC5508712
- DOI: 10.1016/j.molcel.2017.04.012
Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1
Erratum in
-
Structure and Dynamics of a 197 bp Nucleosome in Complex with Linker Histone H1.Mol Cell. 2017 Jun 1;66(5):729. doi: 10.1016/j.molcel.2017.05.018. Mol Cell. 2017. PMID: 28575663 No abstract available.
Abstract
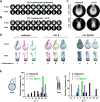
Linker histones associate with nucleosomes to promote the formation of higher-order chromatin structure, but the underlying molecular details are unclear. We investigated the structure of a 197 bp nucleosome bearing symmetric 25 bp linker DNA arms in complex with vertebrate linker histone H1. We determined electron cryo-microscopy (cryo-EM) and crystal structures of unbound and H1-bound nucleosomes and validated these structures by site-directed protein cross-linking and hydroxyl radical footprinting experiments. Histone H1 shifts the conformational landscape of the nucleosome by drawing the two linkers together and reducing their flexibility. The H1 C-terminal domain (CTD) localizes primarily to a single linker, while the H1 globular domain contacts the nucleosome dyad and both linkers, associating more closely with the CTD-distal linker. These findings reveal that H1 imparts a strong degree of asymmetry to the nucleosome, which is likely to influence the assembly and architecture of higher-order structures.
Keywords: X-ray crystallography; chromatin; cryo-EM; histone H1; hydroxyl radical footprinting; linker histone; nucleosome; protein-DNA crosslinking.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




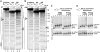
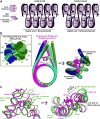
References
-
- Abrishami V, Vargas J, Li X, Cheng Y, Marabini R, Sorzano CO, Carazo JM. Alignment of direct detection device micrographs using a robust Optical Flow approach. J Struct Biol. 2015;189:163–176. - PubMed
-
- Allan J, Hartman PG, Crane-Robinson C, Aviles FX. The structure of histone H1 and its location in chromatin. Nature. 1980;288:675–679. - PubMed
-
- Allan J, Mitchell T, Harborne N, Bohm L, Crane-Robinson C. Roles of H1 domains in determining higher order chromatin structure and H1 location. J Mol Biol. 1986;187:591–601. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

